The Vernier Go Wireless (Bluetooth) pH sensor
Go Big with Vernier’s Go Wireless pH Sensor
pH, like time and temperature, is a physical characteristic that is tossed around daily in science class but rarely understood on a deeper level. In short, time is a measurement of change; temperature is a measurement of relative motion; and pH is the negative log of the activity of the hydrogen ion in an aqueous solution.
Mostly we use
pH for in science eduction as a physical indicator of something. The pH number reflects how close or far a solution is from neutral, or how acidic or basic a solution is.
Mind your Ps and Hs
It’s still a hot debate in such circles as to whether or not p stands for power, but the H as the chemical symbol for Hydrogen is not in question. The p could also stand for potential, or it could have been as simple as the reference solution used way back before pH was known as pH, and the p was just a solution name as in P and Q. Perhaps we’ll never know.

The wirelessness of the sensor allows quality measurements to be made locked behind a protective blast shield and inside a vent hood.
Before diving into what the sensor has, let’s address the elephant in the room, namely what this sensor does not have. In short, the sensor lacks a wire in which to plug it into an interface. The figurative “wire” for this sensor is the Bluetooth radio channel of most modern devices such as the iPad. The radio, a 2.4 GHz
Bluetooth 4.0, Low Energy connection to be exact, runs off a 100 mA lithium-polymer battery in this case.

Measurements are made safely behind glass with no cords to challenge the integrity the venting system.
A Hard Day’s Night
The battery charges in about two hours from it’s USB coupler and is designed to work for three solid days transmitting one sample per second. If your work is not done in that time, you can continue to use the sensor with it plugged into the USB charging cable.
The range of this single junction sealed, gel-filled, Ag/AgCl pH sensor is from 0-14, which, by the way, is not the full range of possible pH values. And the sensor is rated to work in temperatures ranging from 5 to 80 degrees C.
Dethethering is a fancy word for eliminating the cord. By going wireless the probe opens some interesting doors to creative and safe exploration and experimentation. One of the big advantages of a wireless probe is the same advantage we experienced with telephones and microscopes went cordless. No longer did we trip over cords, knock over containers when moving the cords, or dredging off the top of counter tops desks with the cord acting like a giant chain stripping the surface down to bare ground.
Stand Back
Wirelessness also provides an element of safety though both increased distance from potential danger, and to hide behind barriers such as chemical hoods. Vernier’s Go Wireless® pH sensor has a maximum range, like most bluetooth devices, of about 30 meters. In my tests that is workable number as long as its downhill and with the wind. Most lab distances will be only a few meters or so, with classroom applications maybe double that. Outdoors, however, the 30m could actually be a noticable limit, but still a far cry from the 1.3m range of the wired version of Vernier pH sensor.
The product description states…
“Vernier Go Wireless® sensors are rugged, general-purpose, wireless sensors you can use to remotely monitor, collect, and analyze data with iPad, Android tablets, and LabQuest 2!” And the manual is available
here.
The pH probe is actually two pieces attached together through a
BNC connector. The top half is an electrode amplifier powered by a rechargeable battery and is a bluetooth radio transmitter. The lower portion of the probe is the pH sensor.

The BNC connector and probe. Using a ubiquitous connector allows the Bluetooth transmitter to be attached to other sensors.

A potential classroom set of potential.
A school pack of eight pH sensors and a
octo-port charging station is available. Having used multiple pH sensors in one environment is valuable, but it is also critical to mark the sensors and name them within the software so you can tell the identical looking sensors apart on the Bluetooth channel list.
If you need help in this department, you can activate a blinking light on the individual probe by using the
Identify feature in the Go Wireless submenu when using a
LabQuest 2, which, by the way, will require the software update of 2.4 or later.

The sensor is floating 4m away with the iPad recording changes in pH as distance from the shore increases.
Flatwater Rafting
To test the wireless capabilities, I made a raft on which to float the probe away from shore. A local pond provided a nice location. The raft was made of closed-cell foam from a computer packing box. Two counterweights were added to provide stability and maintain the upright position like the keel of a sailboat. Using kite string to for recovery, and stick to launch the boat out about four meters -a far cry from the 30m Bluetooth range, the test was on.

The float with both the pH sensor and Go Wireless temp sensor.
Although the pH increased a few tenths between the shore and 4m out to sea, my hypothesis was that the
huge quantity of duck and goose feces near this particular shore due to local (and firmly discouraged) feeding of the birds would change the pH compared to water further away. The float can carry two Go Wireless sensors, but at the moment only one can be accessed by the App at a time. However, I am told that that will change later this year and multiple sensors will be able to collect and graph data at once.

An island of pH sensor. Floating about 4m from it’s iPad homebase, the platform can transmit one data point per second for 72 hours.
pH is not a huge concern compared to coliform bacteria and other issues with large concentrations of goose poop. Where this all comes together with the wireless sensor is that some water sampling has dangers beyond the classic educational measurements outside of college microbiology. In a case I mentioned in a
prior Science 2.0 post, traditional water quality measurements were collected even though the sample pond was about 500m directly downstream of a densely populated Shanty Town. Human waste was no doubt a significant component in the water meaning that much more formal safety precautions should have been employed during water sampling.

10 meters down to the water.
Radio Fence
Another exploration lowered the float with the Go Wireless pH sensor down ten meters off a bridge onto a river. I used a laser rangefinder to get the exact distance to between the iPad and the sensor. Ten meters on the nose, or one full second of falling according to Newton. All worked fine as long as the iPad was held over the water. Even the slightest amount of bridge metal between the two cut the signal.
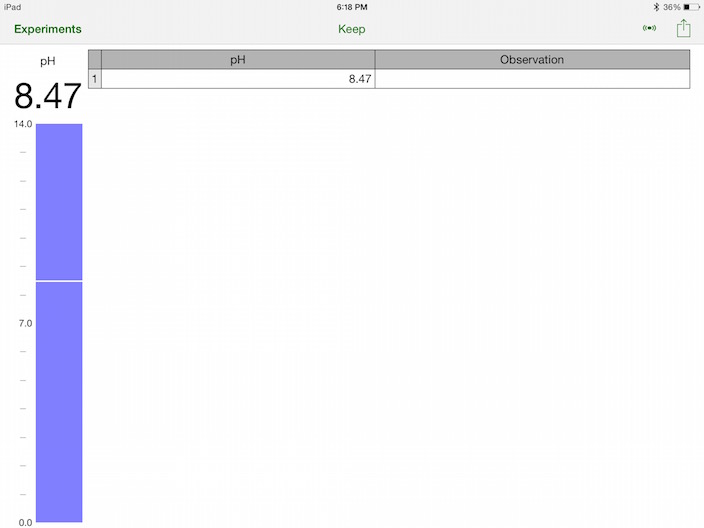
The river’s pH as recorded from the iPad screen running the Vernier App named Graphical.
The river was observed to be about pH 8.5 which I thought a little high. After some research, it seems that the 8.47 reading was perfectly in line with a healthy river, but the pH varies throughout the day. I took my reading at 6:18pm which, according to
this website would put the 24 hour peak pH at mid afternoon, or just a few hours before when I took my reading.
Advanced Simplicity
In the end, the potential of using wireless sensors such as the Go Wireless pH will make data collection easier, faster, safer, and, whether by design or not, much more creative.