Science 101
Q: Why Can I See My Breath on Cold Days?
A: Moisture in the air is normally a gas. We call it water vapor. When moist air cools off, the water vapor in the air can change from a gas to a liquid, resulting in countless tiny droplets of water. That’s what fog is! So when you exhale on a cold day (Figure 1), the water in your moist breath changes from a gas—water vapor—to liquid droplets, which form a fog.

You probably know the terminology involved here, although your students might not. Just to review, vapor is another word for a gas. When a gas changes to a liquid—like water vapor forming liquid droplets, creating a fog—that’s called condensation. So water vapor condenses into liquid water when it cools. At the lower grades, you don’t have to use all these terms; you can just talk about the water in the air changing from a gas to a liquid. We’ll look at a number of examples of this phenomenon, some of which students can observe at home.
When water condenses on a cold soda can, some people say that “the can is sweating.” However, that’s very misleading, since it makes it sound like the moisture is coming from the can. When you sweat, water comes out from inside of you. But with the cold soda can, the water comes from the air. So it’s like the air is sweating! In short, don’t use the word sweat when talking about condensation.
If you ever want to confirm that a sleeping or unconscious person is actually breathing, hold a small mirror up to their mouth and nose. After a moment, you should see fog on the mirror. That’s because water vapor from their breath condenses on the cooler mirror.
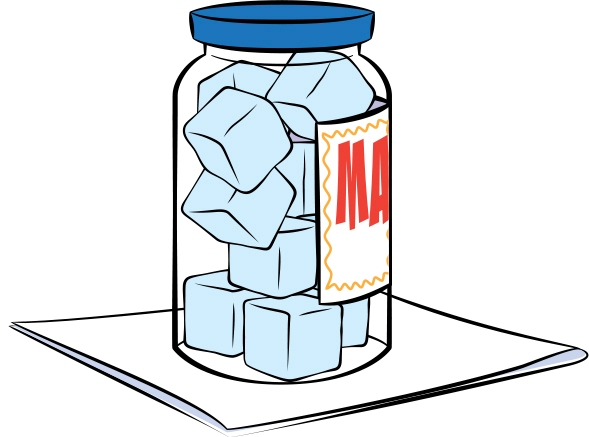
One way to demonstrate condensation is to put ice in a jar with a screw-on or other tightly fitting lid (see Figure 2). I’ve done this with an old (clean and dry) mayonnaise jar. Fill it with ice, and place it on a paper towel. An hour or two later (longer if it’s a less humid day), ask students what they observe. They should see that the outside of the jar is now wet! Maybe the paper towel under the jar is a little wet too. Ask them where that water came from. If they say it’s from the ice in the jar, point out that the lid was tightly screwed on, so there was no way for water to escape from inside the jar. Eventually someone might suggest that the water came from the air, which is correct. There are molecules of water in the air in the form of a gas. When the air touches the cold jar, the water molecules slow down and collect into tiny droplets of liquid water. In other words, water condensed out of the air.
You can also see this when you take a can of soda out of the refrigerator. After a while, the outside of the can becomes moist from water that condensed out of the air (see Figure 3). If it’s a particularly humid day, you might get enough water condensing that it drips down the can and forms a little puddle under it. That’s why we use coasters to protect our nice tables.

Sometimes large masses of air cool off, and when that happens water vapor condenses out of the air and forms—you guessed it—clouds! (See Figure 4.) A cloud is essentially a whole lot of fog way up high. And, you could say that when it’s a foggy day, there’s a big cloud down near the ground. So if you’re in fog, you’re in a low-lying cloud. Again, a cloud is just a place where there are innumerable droplets of (liquid) water. When those water droplets accumulate enough water, they become too heavy to remain suspended in the air and they fall to the ground as rain (or, if it’s cold enough, snow or hail).
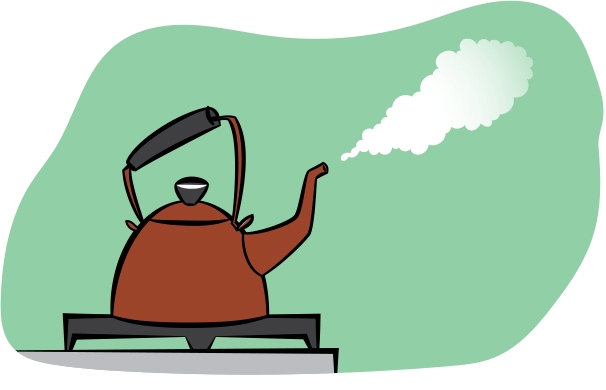
Note that water vapor is invisible. It’s only after that vapor condenses into liquid water droplets, that you can see the water as fog or mist. Try this: Get one of those whistling tea kettles. Get the water in it boiling vigorously so that the steam is coming out very fast. If you look closely (see Figure 5, p. 68), you can see a small gap between the opening in the kettle and where you start to be able to see the steam. In that gap, the water is a gas and therefore invisible. Only after it cools enough for water droplets to form do you see the steam streaming outward.
See Condensation at Home
Since the theme of this issue of Science and Children is “Take Home Science,” here are ways students can see condensation at home.
- Take a hot shower! After you’re all nice and clean, step out of the shower and look at the mirror. See how foggy it is? Where did all that moisture on the mirror come from? There was lots of water vapor in the air, and when the air hit the cool mirror, the water vapor changed from a gas to a liquid and formed a layer of liquid water droplets on the mirror.
- With assistance from an adult, boil some water. When the water is rapidly boiling, you’ll see a white mist (steam) rising from the pot. Where does that come from? There are two steps to creating that mist. The first is that, by heating the water, you’re causing some of it to change to a gas—water vapor. Then, in the cooler air above the pot, some of that water vapor condenses into tiny water droplets, forming the mist (or fog or steam) that you see above the pot.
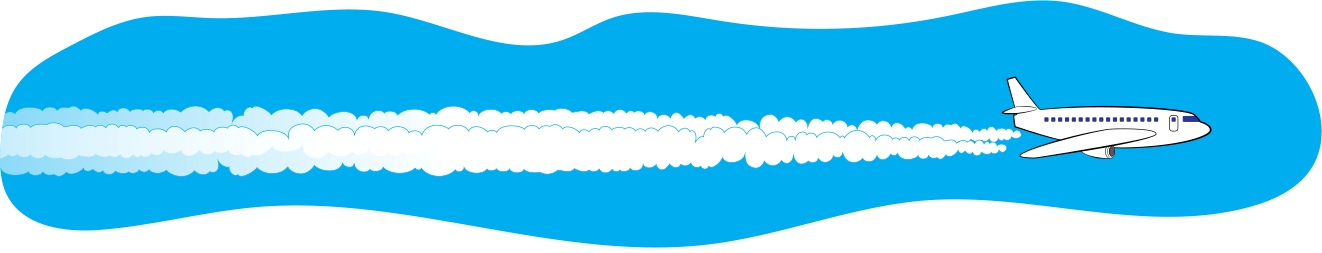
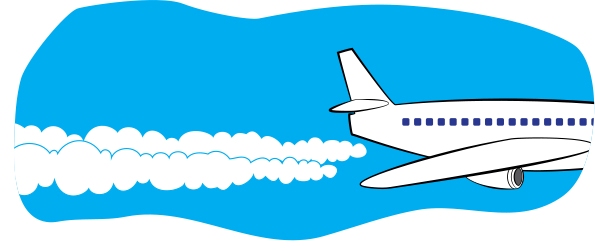
If you wear eyeglasses and get too close to the pot of boiling water, your glasses might fog up. That’s condensation too! Test for Breath If you ever want to confirm that a sleeping or unconscious person is actually breathing, hold a small mirror up to their mouth and nose. After a moment, you should see fog on the mirror. That’s because water vapor from their breath condenses on the cooler mirror. Misconception Alert When water condenses on a cold soda can, some people say that “the can is sweating.” However, that’s very misleading, since it makes it sound like the moisture is coming from the can. When you sweat, water comes out from inside of you. But with the cold soda can, the water comes from the air. So it’s like the air is sweating! In short, don’t use the word sweat when talking about condensation. - Look up in the sky on a sunny day. One thing that you’ll probably see is a cloud (discussed previously). Also, you might see an airplane with a trail of white mist behind it, called a contrail (see Figure 6). The word contrail is short for “condensation trail.” A contrail is not smoke from the engine. It is formed when the water in the jet engine’s exhaust mixes with the outside cold air and condenses and freezes into ice crystals. Contrails are actually a type of cloud. It’s similar to the white exhaust that you might see right after a car’s engine is started on a cold day. FIGURE 6 Water vapor in an airplane’s exhaust condenses to form water droplets, creating a long cloud called a <i>contrail</i>.
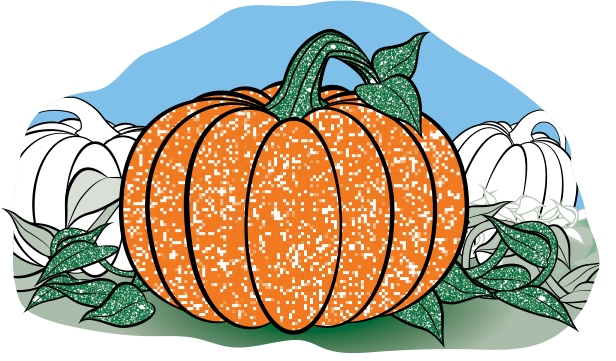
Remember the whistling tea kettle with the gap in the stream of steam where the water vapor had not yet condensed into visible droplets? Sometimes you can see the same effect when you look closely at airplane contrails. There’s a gap between the plane and the contrail where the water vapor has not yet condensed into a visible contrail (see Figure 7). - On a cold day in the winter, when you first get up in the morning, look outside at the grass or leaves. Do you see the morning dew? Overnight, as the air became cooler, water vapor condensed out of the air, making the grass wet. If it’s cold enough, this water will freeze, and you’ll see frost on the grass (or frost on the pumpkin—see Figure 8). You might also see that water has condensed on car windows and then frozen. Time to scrape the ice off the car windows!
- Especially on rainy days (when the air is already very humid), your breath inside a car can cause the windows to fog up. The car windows are cooler, so when the moist air touches the cold windows, water condenses on the glass, making the windows appear foggy.
Remember that condensation is the opposite of evaporation. When water evaporates, it changes from a liquid to a gas. When water condenses, it changes from a gas to a liquid.
Do you teach about the water cycle? When the water droplets or ice crystals in clouds become too big and heavy, they fall down as precipitation — rain or snow. Rain and snow soak into the ground, drain into rivers, and fill our reservoirs. Water on the ground also evaporates, turning back into water vapor, to rise in the air and condense again to form more clouds. So, if you’re ever wondering what to do on a rainy day, talk about rain—or condensation, precipitation, evaporation…and how lucky we are to be on a planet with liquid water. No other planet in the solar system has that. But that’s a topic for a future article. Stay tuned, and never stop learning!
Matt Bobrowsky is the lead author of the NSTA Press book series, Phenomenon-Based Learning: Using Physical Science Gadgets & Gizmos. You can let him know if there’s a science concept that you would like to hear more about. Contact him at: DrMatt@msb-science.com