feature
Keeping Cool With SageModeler
Engaging Students in Systems Thinking and Computational Thinking Through Modeling
The Science Teacher—March/April 2022 (Volume 89, Issue 4)
By Jonathan Bowers, Daniel Damelin, Emanuel Eidin, and Cynthia McIntyre
The COVID-19 crisis has demonstrated the importance of being able to understand complex computational models for everyday life. To make sense of the evolving predictive models of the COVID-19 pandemic, global citizens need to have a firm grasp of both systems thinking (ST) and computational thinking (CT).
Systems thinking is the ability to understand a problem or phenomenon as a series of interconnected elements that produce emergent behaviors (Meadows 2008; Pallant, Lee, and Pryputniewicz 2012). For example, scientists can use ST to examine how different elements of human behavior (social distancing, mask wearing, hand hygiene, etc.) impact the broader system of human interactions that determine how fast the pandemic will spread. However, given the complexity of the pandemic, ST alone is insufficient for constructing or interpreting a meaningful model. To create accurate models, scientists need to use computational thinking. CT involves decomposing a problem into quantifiable elements represented in an algorithmic form that can be interpreted or calculated by either a computer or a person (Sneider et al. 2014; Wing 2006). Such computational artifacts allow users to set different initial conditions and see a range of results and potential solutions. Additionally, refining computational artifacts via testing and debugging as new data becomes available is a hallmark of CT. Not only are ST and CT complementary and critical for constructing pandemic models, they are also essential for understanding a host of other scientific phenomena—from ecosystems to climate change and more.
Although both ST and CT have been increasingly identified as important science practices and crosscutting concepts through the Next Generation Science Standards (NGSS Lead States 2013), there remains a lack of useful learning tools to support teachers and students in ST and CT (Grover and Pea 2017). One new framework seeks to support student engagement in ST and CT by contextualizing these two types of thinking within the practice of constructing computational models (Figure 1) (Damelin, Stephens, and Shin 2019). This framework explores how various aspects of ST and CT are embedded within different modeling practices, as well as how these modeling practices can help students as they construct, revise, and use computational models. In this article, we explore how students interact with the various aspects of ST and CT as they build and refine computational models using SageModeler (a free web-based semi-quantitative system modeling application) in a chemistry unit on evaporative cooling (see Online Connections). Through the examples presented here, we illustrate ways in which this framework can inform curriculum design and pedagogical practice.
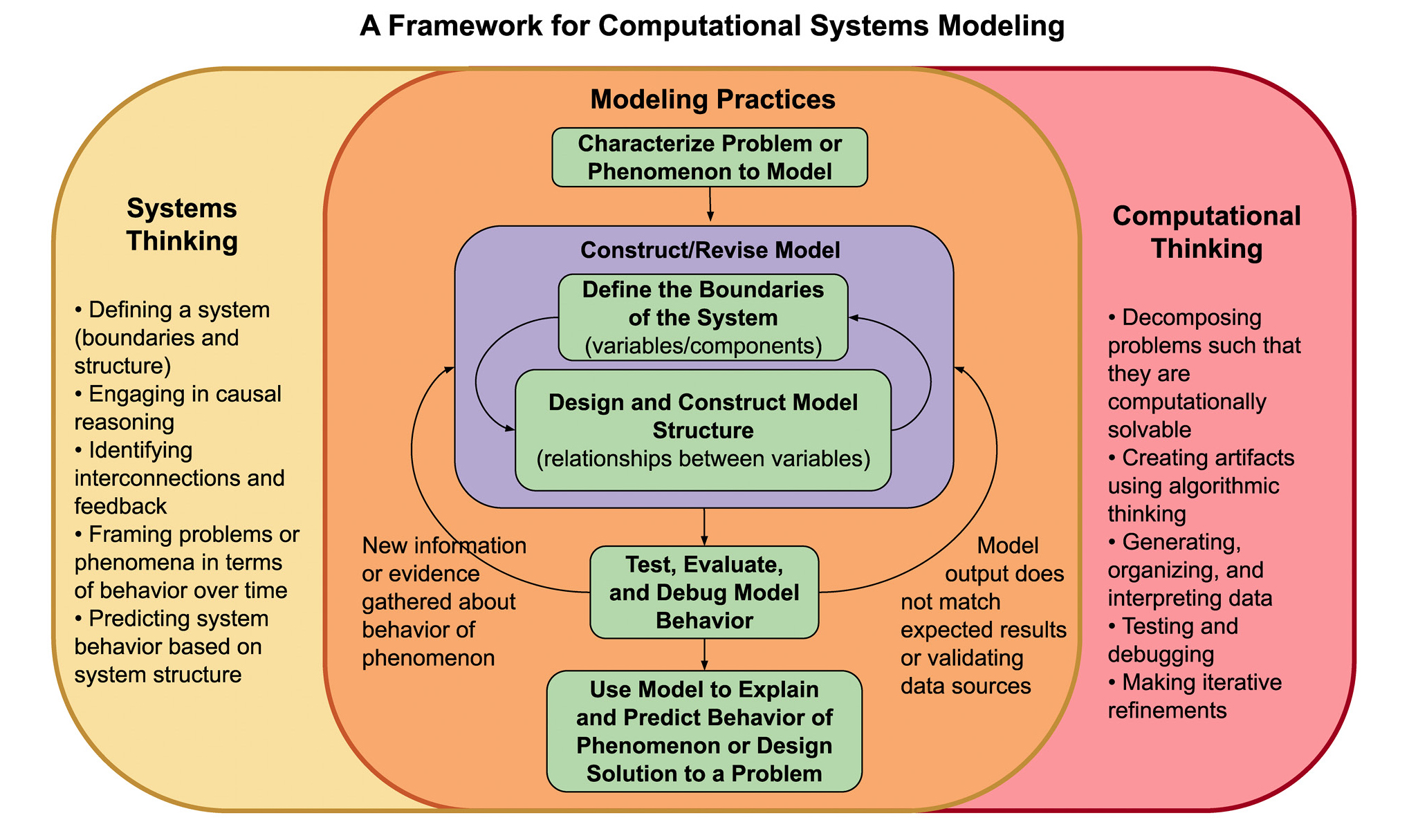
ST and CT through modeling framework.
The framework demonstrates the various aspects of systems thinking (yellow) and computational thinking (red) that support students as they engage in the core computational modeling practices (green).
Evaporative cooling
Evaporative cooling describes the phenomenon that occurs when liquids evaporate, and in the process, the temperature of the liquid and whatever it is touching decreases. This occurs because thermal energy is used to overcome the intermolecular forces attracting the molecules close together, keeping the substance in a liquid state. When the molecules spread apart forming a gas during the evaporation process, thermal energy is converted to potential energy, and the temperature decreases.
We developed a curriculum unit on evaporative cooling consisting of five investigations aligned with the disciplinary core ideas in HS-PS1 (Matter and Its Interactions) and HS-PS3 (Energy), the two science practices of developing and using models and using computational thinking, and the crosscutting concept of systems and system models specified in the Next Generation Science Standards (NGSS Lead States 2013). The investigations were designed using a project-based learning approach (Krajcik 2015; Krajcik and Shin 2014), which includes pursuing a solution to a meaningful question, exploring phenomena using scientific practices, and engaging in collaborative activities to find solutions.
Brief descriptions of all unit investigations—fashioned around the driving question “Why do I feel colder when I am wet than when I am dry?”—can be found in Table 1 (see Online Connections). While students engaged in ST and CT through modeling throughout the unit, our examples primarily come from the first two investigations as the focus of this article is to demonstrate how appropriately designed curriculum activities can support students in modeling practices using systems thinking and computational thinking.
Characterize problem or phenomenon to model
Students begin Investigation 1 by doing a hands-on experiment, placing small amounts of various contact-safe liquids (water, rubbing alcohol, acetone, and oil) on their arms using bulb pipettes. They take notes about how each liquid feels on their skin, which liquids feel colder than others, and which liquids evaporate faster than others. In small-group discussions, students share their observations with one another. Then the teacher introduces the unit’s driving question: “Why do I feel colder when I am wet than when I am dry?” The students then participate in small-group discussions, where they reexamine the phenomenon through the lens of this driving question. After students examine the key ideas of the driving question, they are encouraged to generate additional questions of their own and post them on a Driving Question Board (DQB). The DQB activity helps students break down the larger driving question into more targeted questions that the teacher can revisit throughout the unit (Touitou et al. 2018). Through the initial experiment and the DQB activity, students start identifying macroscopic aspects of the phenomenon (type of liquid, heat of the hand, evaporation rate, etc.) and engage in the ST aspect of defining a system and the CT aspect of decomposing problems such that they are computationally solvable.
Construct/revise model
The next modeling practices—define the boundaries of the system and design and construct model structure—go hand in hand and together constitute the overarching practice of construct and revise a model. Students begin by creating model illustrations with an online drawing tool, using the ST aspect of defining a system and the CT aspect of decomposing problems as they define the boundaries of the system. Students’ illustrations and accompanying text explanations are early sensemaking representations of evaporative cooling in which they begin to consider the underlying mechanisms behind the phenomenon (Figure 2). In particular, students describe how the hand’s heat causes the liquid molecules to move faster—allowing some to evaporate—and that loss of heat makes them feel colder.
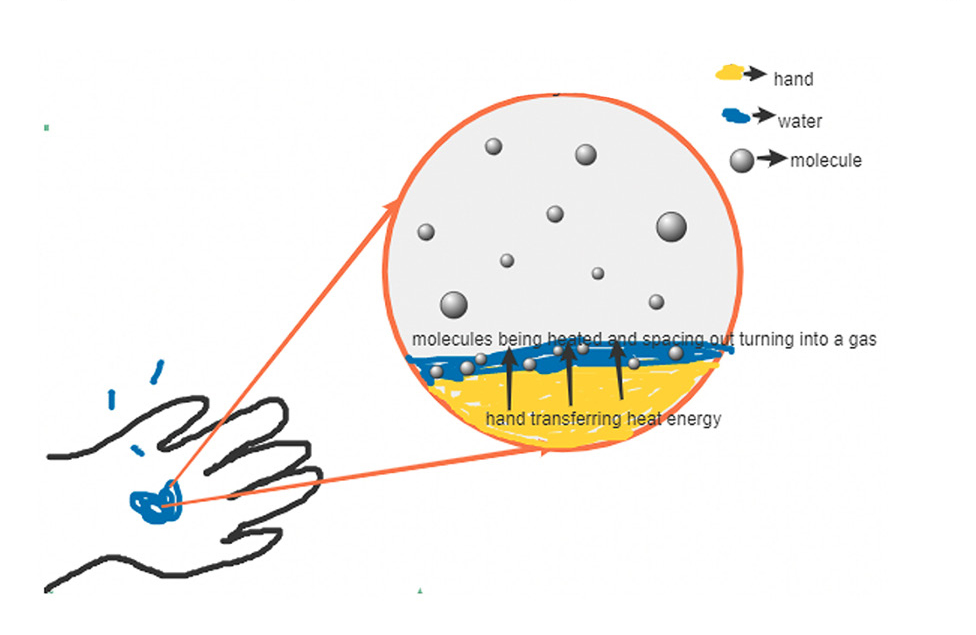
Student model illustration of evaporative cooling.
An example of a student’s initial model illustration of evaporative cooling and a written explanation of their drawing. Student written explanation: The body heat slowly but surely transfers heat to the liquids causing the molecules to speed up and space out which eventually causes them to change from the liquid to the gas stage, aka evaporation.
Additional classroom discourse surrounding these illustrations helps students in defining specific elements as key variables that can be modeled computationally in SageModeler, including evaporation rate and heat of liquid. By facilitating discussion around the evaporative cooling process and the key variables needed to model this process, these illustrations not only help students participate in the modeling practice of define the boundaries of the system, but also assist with the cognitive shift needed to design and construct model structure using SageModeler system modeling software (see Online Connections). Students create their initial computational models in SageModeler at the end of Investigation 1 by engaging in the ST practice of engaging in causal reasoning and recognizing interconnections and identifying feedback and the CT practice of creating artifacts using algorithmic thinking.
SageModeler takes a semi-quantitative approach to modeling. Students begin by adding variables to their workspace, using a simple drag-and-drop interface. For effective modeling, these variables must be quantifiable (i.e., able to vary on a low to high scale), and students need to convert objects (such as “hand” or “water”) into measurable variables such as “temperature of hand” or “amount of water.” Next, they set causal relationships between variables by linking them. The directionality of relationship arrows shows causality (e.g., an increase in “temperature of hand” causes an increase in “evaporation rate”), and the color of lines shows the general trend of the relationship (red indicates positive correlation, blue indicates negative correlation). Finally, students can adjust the magnitude of these causal relationships using the relationship tab.
Test, evaluate, and debug model structure
Throughout the unit, students test, evaluate, and debug model structure as they carry out additional hands-on investigations into different aspects of evaporative cooling. For example, in Investigation 2, as students explore the investigation-level question, “Why do some liquids ‘stick together’ more than others?” they are introduced to how differences in the Intermolecular Forces (IMFs) of various liquids affect their behavior. They conduct an experiment to determine how many drops of liquid (water, acetone, and rubbing alcohol) can fit on a penny. Using a pipette, they add individual drops of a liquid until the surface tension breaks, causing the “bubble” of liquid to burst (Figure 3). Students repeat this experiment three times with each liquid, comparing their results with their initial observations of evaporative cooling. They learn that the liquids that felt cooler and evaporated faster had less surface tension than the others. Such surface tension is indicative of the strength of the IMFs of the liquid particles and is a meaningful macro-level indicator of this important chemical property.

Example from surface tension experiment.
(A) Example of a penny holding several drops of liquid in a “bubble”; (B) the coin after enough drops have been added to break the surface tension of the “bubble” of liquid.
Next, students explore the relationship between the strength of attraction between particles, or IMFs, and the state of matter using an interactive simulation (Figure 4). After discussing this experiment and simulation and revisiting the Driving Question Board, students revise their models, using these additional sources of evidence to validate or refute specific components of their models and engaging in the ST aspect of framing problems in terms of behavior over time and the CT aspect of making iterative refinements.
In addition to modifying their models based on new experiences and conceptual knowledge, students use SageModeler to test and debug their models. The “simulate” function within the software allows students to generate output from their models as they adjust the amounts of each input variable to see how it impacts other variables and the model’s behavior (Figure 5A). Students can also explore specific relationships between two variables by generating graphs, and compare their graphs with both external data and their conceptual understanding. For example, a student might notice that their model incorrectly predicts that liquids with higher IMFs evaporate more quickly. They can then change their model by redefining the relationship to better reflect their experimental results (Figure 5B).
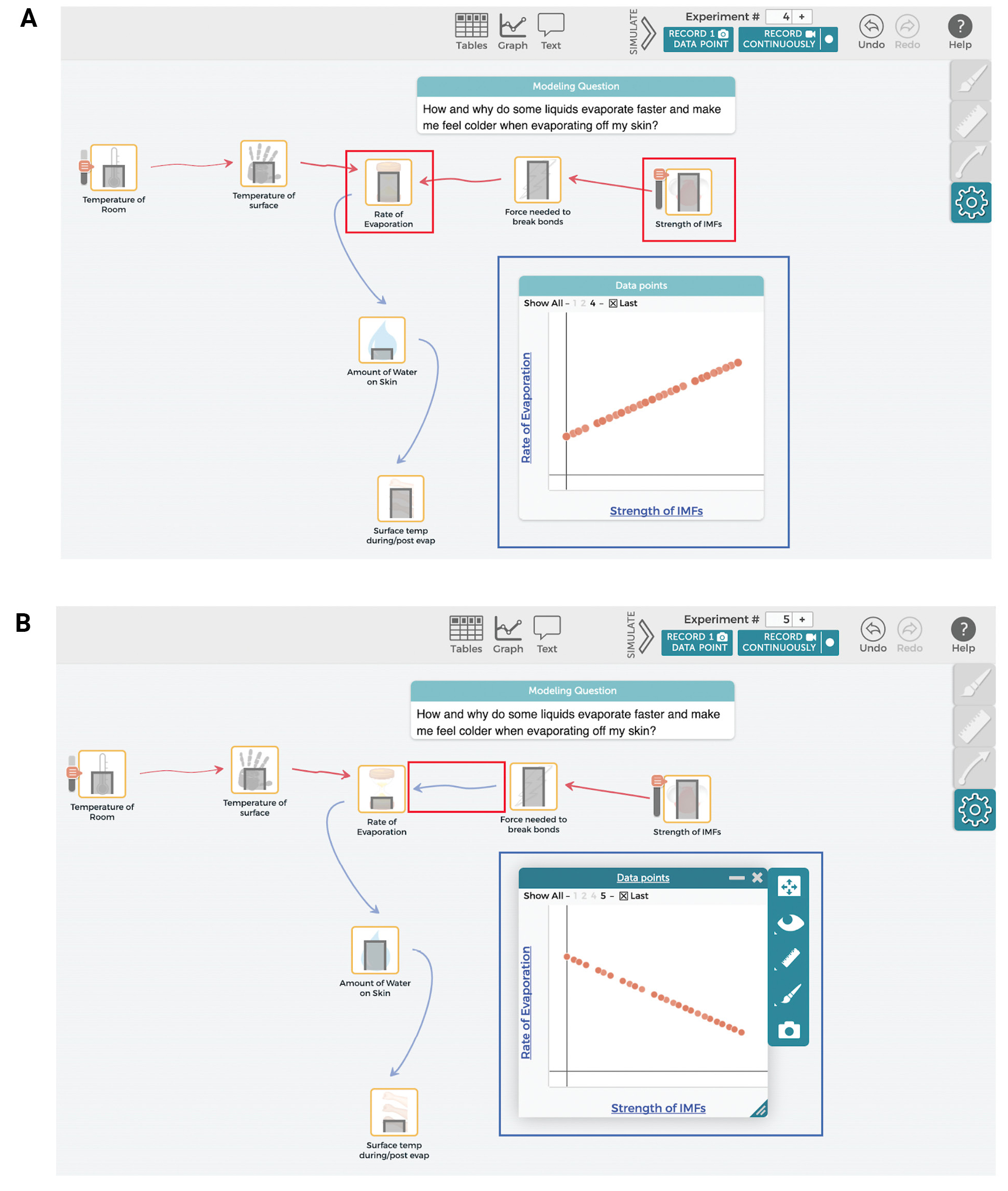
Testing and debugging using SageModeler.
(A) The simulate function is used to manipulate the input variable of “Strength of IMFs” to determine its impact on the variable “Rate of Evaporation”; (B) a line graph was also made to further clarify the relationship between “Strength of IMFs” and “Rate of Evaporation.
Peer critique is another essential resource for helping students improve the usefulness of their model or identify any flaws. Students meet in small groups to share and discuss their models, guided by our model design guidelines (which is also used by teachers to provide formative assessment and feedback on student models) (Figure 6). Such peer critique helps students identify aspects of their models that can be improved while also generating new ideas from examining the work of others. Through these varied approaches for supporting the modeling practice of test, evaluate, and debug model behavior, students are able to participate in the ST aspects of recognizing interconnections and feedback and engaging in causal reasoning, as well as the CT aspects of generating, organizing, and interpreting data, testing and debugging, and making iterative refinements.
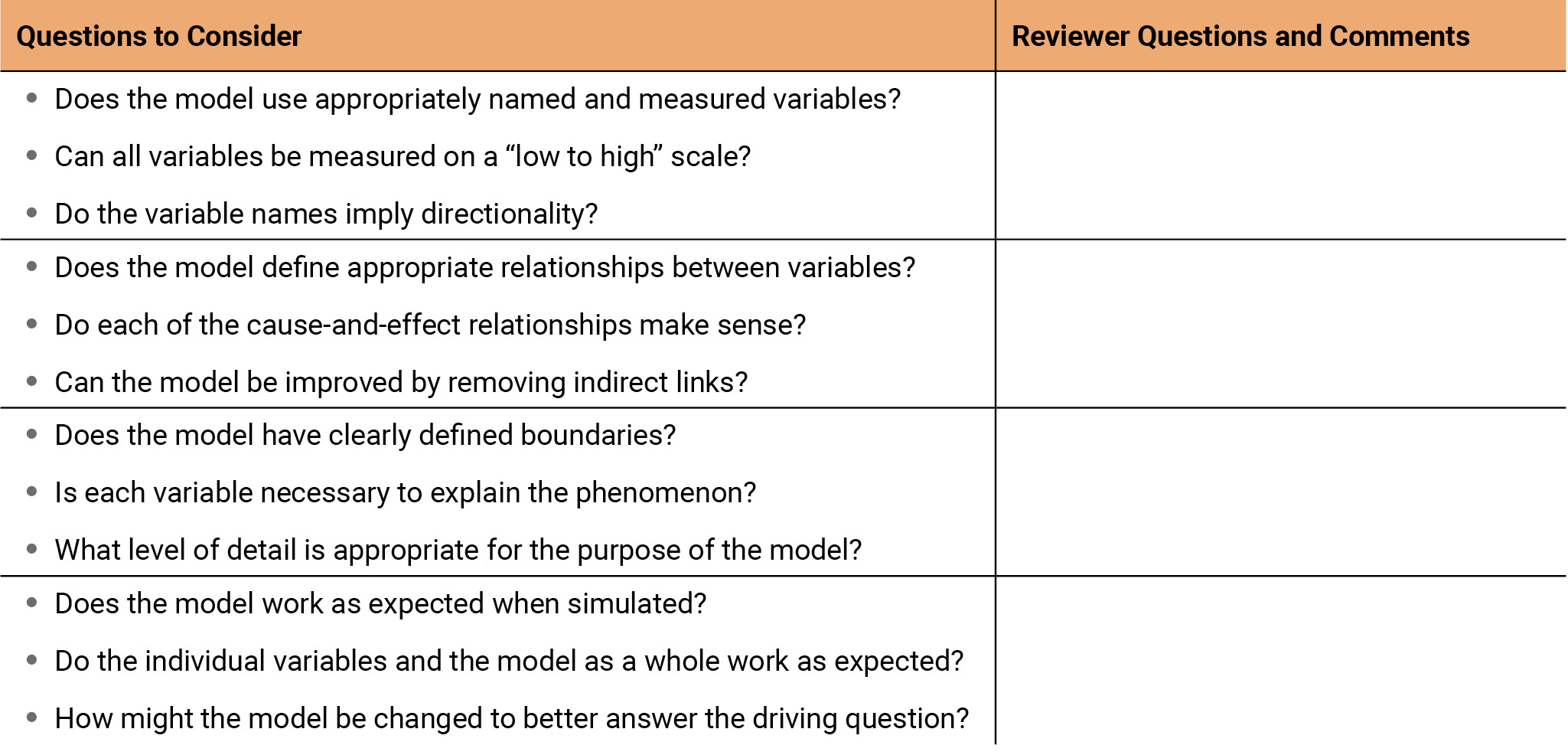
Model design guidelines.
The model design guidelines help scaffold students in providing meaningful feedback during peer critiques and is used by teachers to provide formative assessment and feedback to students as they construct and revise their computational models.
Use model to explain and predict behavior of phenomenon or design solution to a problem
At the end of the unit, students return to the Driving Question Board to address any final questions they have regarding evaporative cooling. A summative assessment task allows students to use model to explain and predict behavior of phenomenon or design solution to a problem. They write an explanation of evaporative cooling that addresses the driving question of “Why do I feel colder when I am wet than when I am dry?” using their models to scaffold their written responses (Figure 7). Students are then encouraged to reflect on the evidence used to create their models and, therefore, reflect on the whole modeling process.
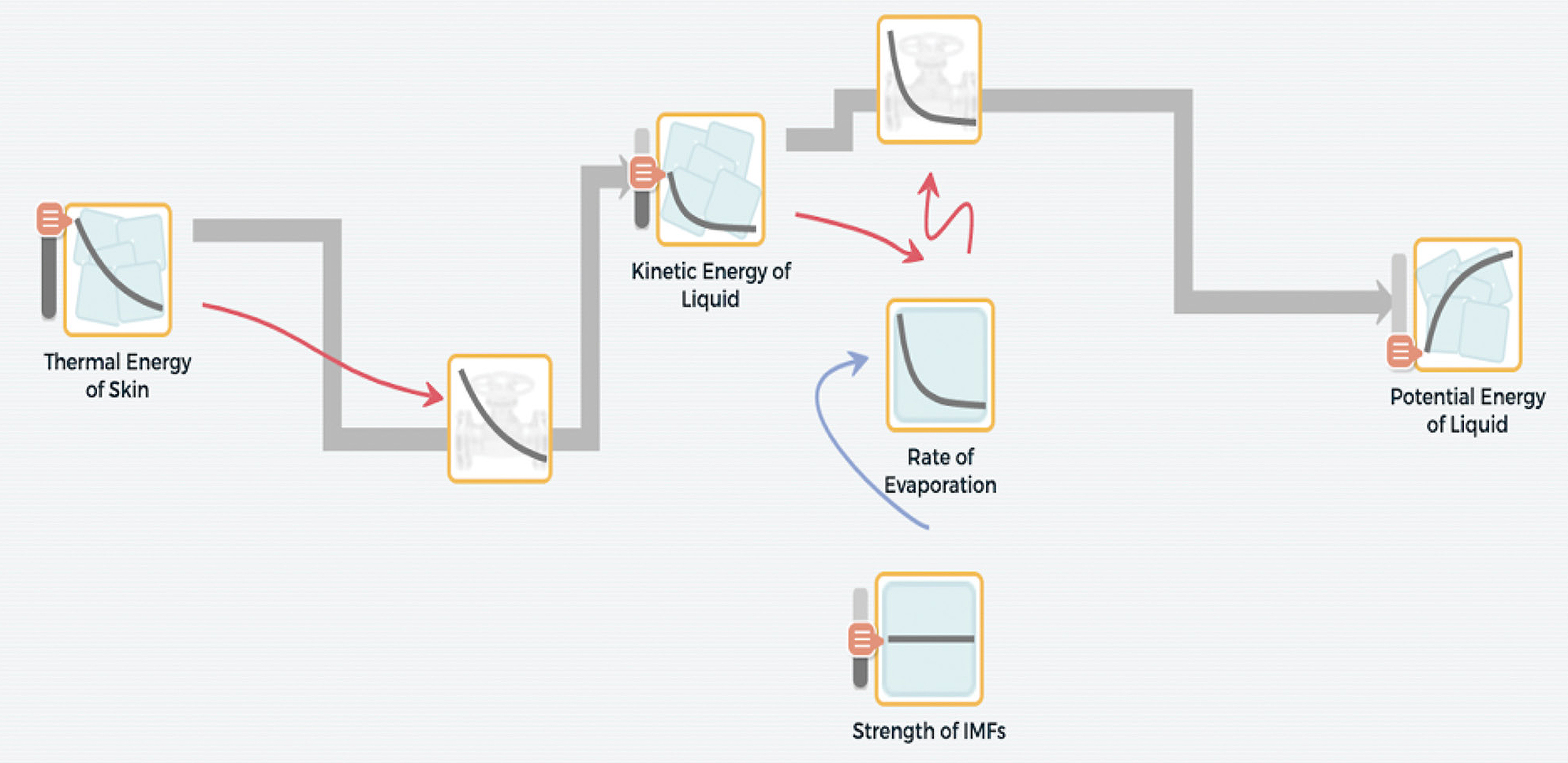
Model-based explanation of evaporative cooling.
An example of a student’s final model along with a transcription of their explanation of evaporative cooling based on their model. Student explanation: This model shows how evaporation causes us to feel colder when we are wet. The thermal energy of our skin makes the liquid warmer (increasing its temperature and kinetic energy). As the liquid particles have more kinetic energy, they will move faster and begin to break free from the liquid and evaporate. Once the liquids are free from these intermolecular bonds, they become gas molecules with high potential energy. Liquids with a higher IMF evaporate slower because it requires more energy to overcome the intermolecular bonds. I included some “feedback” relationships because the more energy there is in the skin, the faster the liquid will heat up and the more kinetic energy in the liquid, the faster the liquid will evaporate.
Conclusion
When students engage in the NGSS practices of developing and using models and using computational thinking, and the crosscutting concept of systems and system models (NGSS Lead States 2013), they have opportunities to apply three-dimensional learning and make sense of scientific phenomena. This article demonstrates how a chemistry unit on evaporative cooling with an embedded system modeling tool called SageModeler can help students in the critical work of exploring and understanding complex problems. The framework for scaffolding students in ST and CT through modeling can be applied to other disciplines—including climate change, ecosystems, population dynamics, forces and motion, and many other topics—to foster student participation in systems thinking and computational thinking through modeling. In doing so, they will build skills to help them solve complex problems of the future. ■
Acknowledgments
This material is based upon work supported by the National Science Foundation under grants DRL-1842037 and DRL-1842035. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Online Connections
SageModeler: https://sagemodeler.concord.org/
Free curriculum unit: https://learn.concord.org/building-models
Table 1: https://www.nsta.org/sites/default/files/journal-articles/TST89-4/Bowers/Table_1.pdf
Connecting to the Next Generation Science Standards: https://www.nsta.org/sites/default/files/journal-articles/TST89-4/Bowers/NGSS.pdf
Jonathan Bowers (bowersj8@msu.edu) is a PhD Student and Emanuel Eidin is a Postdoctoral Researcher at Michigan State University, East Lansing, MI; Daniel Damelin is a Senior Scientist and Cynthia McIntyre is Director of Communications at The Concord Consortium, Concord, MA.
Computer Science Engineering Instructional Materials STEM Teaching Strategies Technology High School


