Disequilibrium
Surface Tension and Surfactants
Science Scope—February 2019 (Volume 42, Issue 6)
By Todd Hoover
In its description of Stability and Change, A Framework for K–12 Science Education (NRC 2012) discusses how systems can be thrown into disequilibrium at varying rates. An example of a quick event causing disequilibrium would be a hurricane disrupting a local habitat. An example of a slower disequilibrium event is the use of pesticides (DDT) over an extended period of time that may harm the development of eggs from certain species of birds such as eagles. This month’s activity shows how soap added to water quickly creates disequilibrium in surface tension.
Engage
Use the “drops on a penny” discrepant event to engage your students on the concept of surface tension. Place a penny, cleaned only with tap water, on a flat surface that can get slightly wet, such as a lab table or student desk. Fill a clear plastic beaker or clear plastic cup with fresh tap water. Ask students to predict how many drops of water will fit on top of the penny without spilling over the edge. Students often predict five or 10 drops.
Explore (part 1)
Using an eye dropper (see Materials), have students place water, one drop at a time, onto the penny while counting the total drops (Figure 1). Students are usually impressed with the number of drops that fit. The number varies depending on conditions. But it takes typically between 30 to 50 drops, depending upon the cleanliness of the penny, the purity of the water, and the size of the water drops.
- two clear clear plastic cups per set
- eyedropper (one per set)
- 200 pennies per set
- liquid dish detergent
- tap water
- if using the alternative assessment, containers of various sizes and shapes

Explain (part 1)
First, describe the surface tension of water to students. Some types of molecules are electrically attracted to other molecules of the same type. This phenomenon is called cohesion. Water molecules are cohesive, causing them to try to “stick” together and clump into drops and pools.The cohesive forces between water molecules are the result of the structure of water molecules. For reasons well beyond the scope of middle school, the oxygen side of water molecules retains a tiny electrical charge that is negative, and the side of the molecule with the hydrogen atoms is slightly positively charged. When two water molecules come close together, the tiny electrical forces cause them to rotate so that the positive side (+) of one molecule is close to the negative side (-) of the other molecule. This causes them to attract to one another and hold together.
Water molecules at the surface of a drop or pool of water feel an attraction toward the water molecules, but not toward the surrounding air. This phenomenon is referred to as surface tension. A water droplet forms a round shape and stays in that shape due to surface tension—the water molecules on the surface are attracted to the water inside the droplet but not with the air. Show students Figure 2 to support the explanation of the surface tension of water.
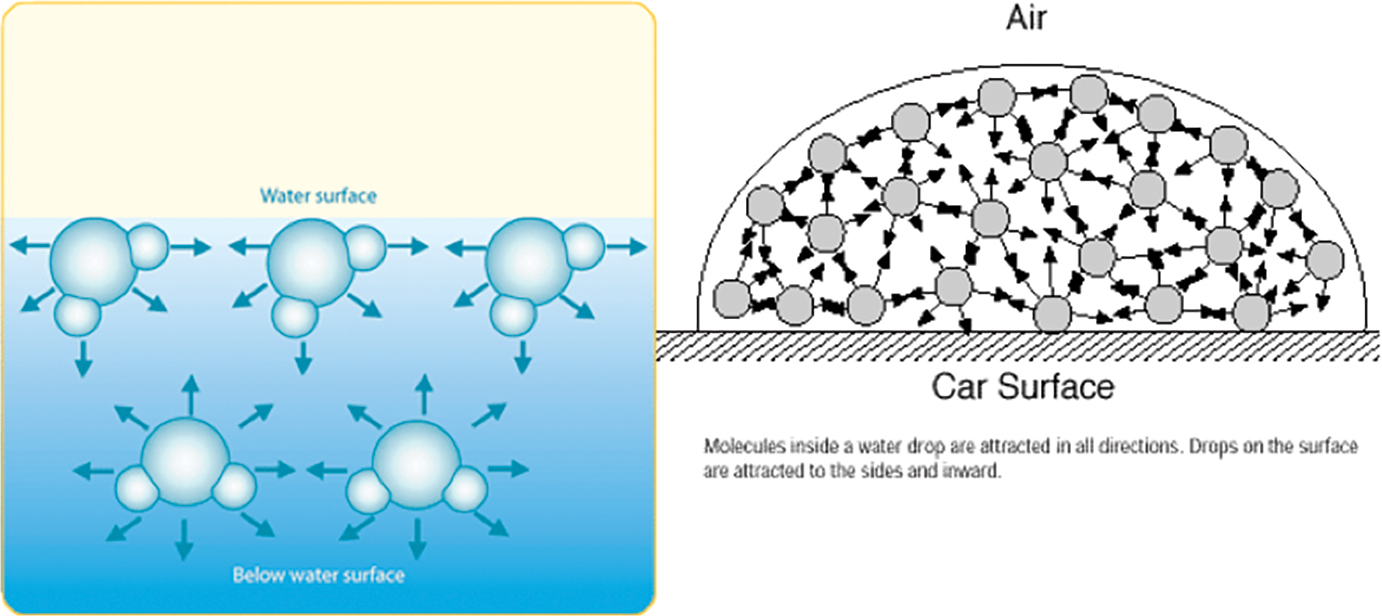
Next, explain the role of surfactants to students. A surfactant is a substance that reduces the surface tension of a liquid. For instance, a small puddle of water on a desk has high surface tension. But adding a surfactant such as dish soap to the puddle would reduce its surface tension and cause the water to spread out more.
Elaborate
Now that students have a basic understanding of surface tension, let’s look at the phenomenon on a slightly larger scale. Fill one small clean plastic cup with tap water to the brim. Add a drop of dish detergent to a second small glass and fill it with tap water completely to the brim, as in the first glass.
Ask students to predict how many pennies they think they will be able to add to each glass before the water overflows. Then, have students test their predictions by gently placing pennies into the water. Students should find that the glass without the detergent (Figure 3) will hold more pennies than the one with detergent added (Figure 4). The number of coins that can be added to the glass without soap varies. But a 473 mL (16 oz.) glass can typically hold 40 pennies, depending upon the cleanliness of the pennies, the purity of the water, and the size of the glass. The glass with soap will overflow after just a few pennies are added.

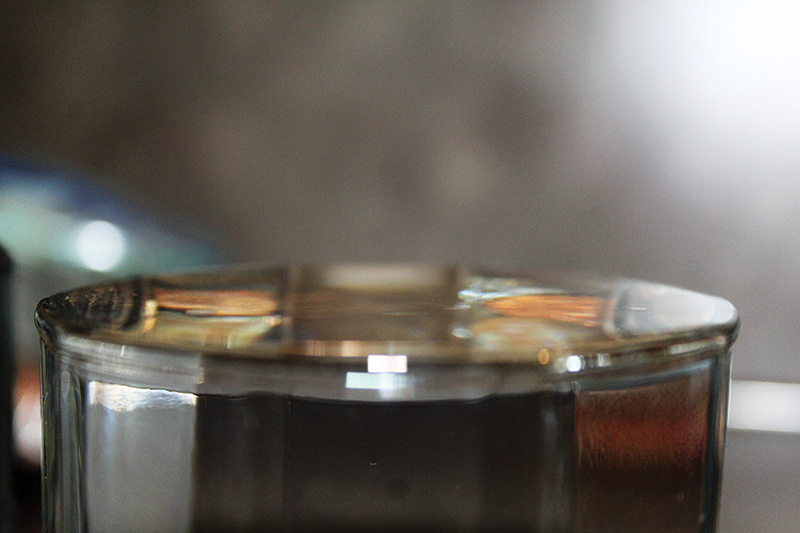
Explore (part 2)
To gain an understanding of why this occurs, have students watch the American Chemical Society video “Surface tension” up to the 3:18 mark (see Resources).
The video explains that the water molecules on the outside of the drop are arranged in such a way that they are being drawn inward toward other water molecules. Because the surface of the water is aligned with air, the water molecules are attracted to one another from side to side. This creates surface tension. But when you add the surfactant—the dish detergent—its molecular structure causes the bonds between the water molecules on the surface to weaken, which decreases the water’s surface tension.
Explain (part 2)
Ask students to demonstrate surface tension of water by representing it with their own bodies. Each student represents a water molecule and the class represents all the water in the glass. You may need to facilitate the process until students are standing in a large group, with each student representing a water molecule.
Keep a couple of students away from the group to represent the dish detergent, who will be added to the water shortly. Have the rest of the students model water using their body, with the oxygen atom being their back, and their hands being the hydrogen atoms. Students should hold their arms out in a V-shape, modeling an actual water molecule, while orienting themselves so that their hands point toward oxygen atoms (touching the back of another student) or away from hydrogen atoms. Remind students to be gentle and courteous of each other while carrying out this entire activity.
The “dish detergent” students should model it by carefully and gently walking through the group to break apart the molecular bonds (students’ hands on other students’ backs) of the water molecules. As the “detergent” students move through, the other students will need to move apart to make room for the detergent (weakening the bonds) or reorient themselves as other molecules also move (breaking and reforming bonds). Discuss with students that prior to adding the detergent, the water molecules were stable. As detergent was added, the molecules were thrown into a state of disequilibrium, causing change. For a more detailed explanation of the concepts of Stability and Change, please see A Framework for K–12 Science Education.
Evaluate
Have students predict what will happen if you return to the original “drops on a penny” activity, but this time use a water-detergent mixture. Next, students predict, test, record, and provide a scientific explanation of their results. Their explanation should mention that the detergent breaks the the electrical forces holding the water together, greatly reducing the surface tension and causing the water to overflow more quickly than when there is no detergent in the water.
As an alternative performance assessment, you could challenge students to develop and conduct an experiment showing how different variables affect the outcome. The teacher would then provide students with larger containers with wide mouths, larger containers with narrow mouths, and smaller containers. Students would test if the same amount of detergent is needed to create the same impact. Students would also determine if the size and shape of the containers affects the amount of detergent that needs to be added to have a similar effect on the surface tension as the one drop of detergent did on the small glass. Students would choose how to record and use their data to write a scientific explanation that explains their results.
Participants should wear indirectly vented chemical safety goggles and gloves during all phases of the activity. Wash hands with soap and water upon completing this lab activity. The teacher should prepare so that students are working in a clear, unobstructed area to minimize spills. Water on tile floors can be slippery. No part of the activity should be ingested. Students should be advised that no roughhousing will be permitted during any portion of the activity.
Interdisciplinary Physical Science Middle School


