Bringing College-Level Protein Analysis to the High School Biology Lab
By Heather Dyc; Fact Checked by Jeff Baker
Posted on 2025-07-16
Disclaimer: The views expressed in this blog post are those of the author(s) and do not necessarily reflect the official position of the National Science Teaching Association (NSTA).
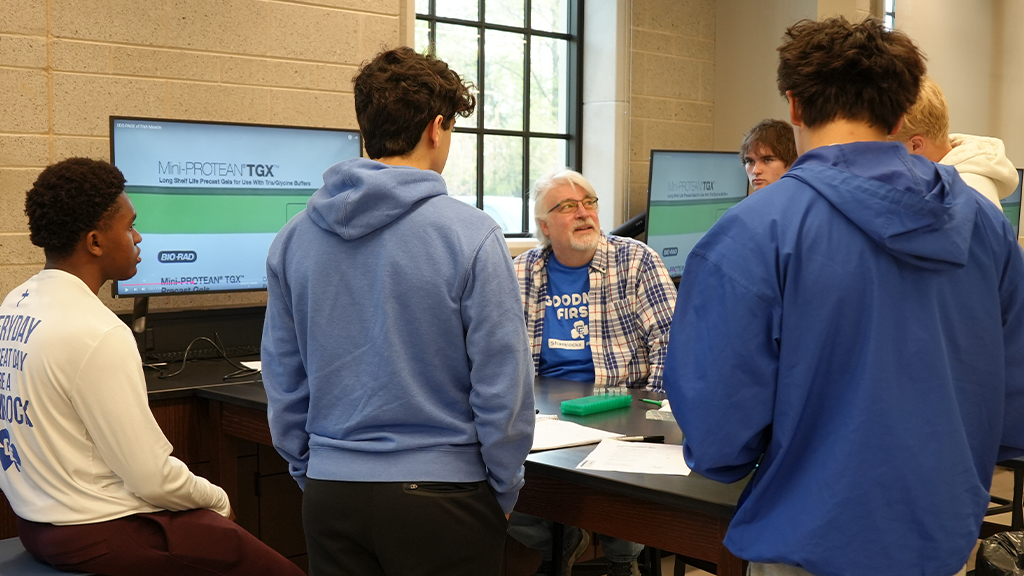
When I entered Jeff Baker’s AP Biology classroom at Catholic Central High School last week, I didn’t expect to find students carefully loading gels and running electrophoresis like seasoned researchers. But that’s exactly what was happening.
It was our school’s first time introducing SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) to the classroom, and I couldn’t help but feel exhilarated. For those of us who haven’t observed a lab in a while, SDS-PAGE is a technique used to separate proteins based on their size—standard fare in university and research settings, but still relatively rare at the high school level.
Baker had organized the lab as part of a unit on molecular biology and evolution. His students examined muscle proteins from five species of fish—Atlantic salmon, rainbow trout, whitefish, Dover sole, and swordfish—using electrophoresis to create what you might call “protein fingerprints.”
Each fingerprint appeared as a unique set of bands on a gel, representing different proteins sorted by molecular weight. The main focus was on myosin light chains, muscle proteins common across vertebrates. By comparing the banding patterns, students could start making evolutionary inferences.
What they saw was compelling: Salmon and trout had nearly identical protein profiles, with whitefish not far behind. Swordfish, on the other hand, stood out as the most distinct, likely the out-group in this evolutionary analysis. Dover sole fell somewhere in between. It wasn’t just a fun visual; it was molecular data painting a picture of evolutionary relationships.
“This is the most effective way I’ve found to demonstrate evolutionary relationships using real data,” Baker told me as he monitored the gel run. “It’s a hands-on method that mirrors what scientists actually do when they’re analyzing genetic and molecular evidence.”
The students’ next step is to construct a standard curve from the gel data. By plotting known protein sizes against their migration distances, they can estimate the molecular weights of the bands they observed. It’s a simple but powerful technique that helps confirm evolutionary hypotheses with quantitative support.
The unit will conclude with the students building their own cladograms—evolutionary trees based on their protein data—and comparing them to established phylogenetic models. It’s one thing to read about fish evolution in a textbook; it’s quite another to see how molecular similarities can map out those relationships in the lab.
What impressed me most was how smoothly the students handled the technical process. The equipment was key, of course. The lab was made possible with a kit from Bio-Rad, a company that sells science education products designed for classroom use. While SDS-PAGE isn’t yet a staple in every high school, kits like these are making it increasingly feasible for science teachers to bring authentic lab experiences to their students.
“It’s definitely a more advanced technique, but the students rose to the challenge,” said Baker. “I think they really appreciated the opportunity to use equipment and procedures that go beyond the usual high school biology curriculum.”
The students weren’t just following the directions for the lab. The room was filled with their questions about protein structure, evolutionary divergence, experimental controls, and the significance of what they were seeing on the gels. They weren’t just learning about molecular biology; they were doing it.
For any science educator considering a deeper investigation of molecular biology or evolution, I’d say this kind of lab is worth exploring. Watching students unravel evolutionary patterns with their own data was an inspiring reminder of what’s possible when we bring real science into the classroom.
Want to hear about news and events from Catholic Central? Follow us on Facebook, Instagram, and LinkedIn.
Heather Dyc is the Communications Specialist at Catholic Central High School, an all-boys private school in Novi, Michigan, where she also serves as editor of CatholicCentral.net. She holds a BA in Journalism and an MS in Nutrition.
Jeff Baker has been an educator for 33 years, with the last 25 at Catholic Central. He earned both his BS (1990) and MAT (1994) degrees from Miami University, briefly pursued a PhD at Northwestern University, and ultimately found his true calling in the classroom.
The mission of NSTA is to transform science education to benefit all through professional learning, partnerships, and advocacy.


