Polymers Course for Small Colleges and Universities
Journal of College Science Teaching—September/October 2018
By Joseph Furgal
This article describes the course design and teaching methodology for a polymer chemistry and applications lecture class specifically aimed at small college and university instruction. This intermediate course for advanced undergraduates and masters-level graduate students focuses on teaching the basics of polymer history, synthesis, and characterization with connections to the core chemistry curriculum in a small-class-size environment and without a textbook. Furthermore, an extensive overview of the applications of polymeric materials gives students a connection to real-life applications. The course includes polymer case studies, informational lessons on realworld objects made of polymers, and demonstrations. Student presentations on how polymers are important to society help connect the course to the world around them. The course is designed to instill the knowledge necessary for students to be successful in a career in polymers. A brief discussion of course reflections and student input is also given.
P olymer science is typically underrepresented in small colleges’ or universities’ chemistry curricula even though almost half of all chemists will work in this field at some point in their careers, especially those colleges with less emphasis on research programs (https://www.acs.org/content/ acs/en/careers/college-to-career/ chemistry-careers/polymers.html). One of the major limitations to adding instruction in polymer education dating back to at least the 1950s is that colleges traditionally stick to the core disciplines of chemistry including organic chemistry, inorganic chemistry, analytical chemistry, physical chemistry, and biochemistry for hiring, course purposes, and degree requirements, even if the professors have a PhD and/or postdoctoral training in other areas including polymers/macromolecules. Even if some of them had been exposed to polymeric materials in their graduate studies, this leaves no motivation to revamp decades’ worth of curriculum, to expand outside their comfort zone, and to incorporate polymer course materials into the current course load or to add dedicated courses (Billmeyer, 1959; Jefferson & Phillips, 1999; Kice, 1959; Stenzel & Barner-Kowollik, 2006). Furthermore, polymer education was not part of many certified/accredited degree programs, including the American Chemical Society (ACS) certified degree program, leaving no motivation for schools to advance curriculum in this area; however, this is finally changing, with the most recent requirements for the ACS certified degree requiring macromolecule/ polymer education (https://www.acs. org/content/acs/en/about/governance/ committees/training/acs-guidelinessupplements.html).
Over the years, a series of efforts have been made to integrate polymer topics within the core subject areas of chemistry, most notably as reported by the polymer core course committee during the 1980s, which discussed the addition of polymer topics to each core area (Core Course Committee in General Chemistry, 1983; Core Course Committee in Physical Chemistry, 1984; Droske, 1995; Howell, 2013; Miller et al., 1984). Recently there has been an effort to stress the teaching of polymer science, and many guidelines have been proposed; however, most were to deaf ears, likely leaving graduates underqualified and underpaid for today’s job market (Carraher & Deanin, 1980; Cavalli, Hamerton, & Lygo-Baker, 2015; Goh, 2013; Hamaide, Holl, Fontaine, Six, & Soldera, 2012; Jefferson & Phillips, 1999; Mahaffy, 2004; Seymour, 1982). The new degree requirements, which include instruction in macromolecules/polymers, leave smaller institutions with a challenge as to how they can meet the new stipulations. These institutions are not likely to have the resources to hire a dedicated instructor for teaching polymers and must find creative ways to incorporate instruction into their current courses or implement something that can be taught by the current faculty. To overcome some of the limitations presented for current faculty, a number of polymer-teaching workshops and free online courses have been implement ed at universities to impart the necessary knowledge needed to include a connection to polymers in professors’ courses (Stinson, 1989; http://www. open.edu/openlearn/science-mathstechnology/science/chemistry/intro duction-polymers/content-section0?active-tab=description-tab). Some small universities may also be able to hire qualified adjunct professors to teach such classes.
A few education articles over the years have suggested various course topics and training methods for polymer chemistry (Carraher & Deanin, 1980; Cavalli et al., 2015; Goh, 2013; Hamaide et al., 2012; Jefferson & Phillips, 1999; Mahaffy, 2004; Seymour, 1982). This article describes the design and teaching methodology of a polymer chemistry course for advanced undergraduates and masters-level graduate students at small, primarily undergraduate institutions (PUIs), a course that could be taught by any savvy chemistry professor outside of polymers, an instructor with a background in polymer chemistry, or a knowledgeable adjunct. This course is perfect for small colleges and universities as it gives students a broad survey of synthetic techniques, correlations to core chemistry subjects, and a review of everyday applications in polymer science.
Course design
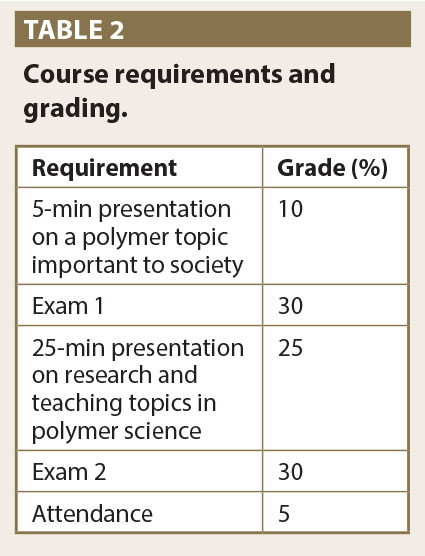
The goals of this course are to give students a broad understanding of the principles of polymer chemistry and applications at an intermediate level with little starting knowledge of what polymers are. This includes developing a basic knowledge of polymer synthetic techniques, characterization, and topics on the many applications of polymeric materials and their connection to other disciplines of chemistry. In detail these goals include developing a global perspective of interdisciplinary issues involved in polymers; learning how to design, synthesize, evaluate, analyze, and implement functional polymeric materials; critical thinking and analysis skills to develop research interests and proposals; and finally, effective communication of ideas both individually and within a group through written and oral communication (Porter, 2007).
This class was designed to use Internet and library resources instead of relying on a single purchased course textbook, which kept costs lower for students (see supporting information at http://www.nsta.org/college/con nections.aspx for instructional materials links). As an instructor, however, textbooks provide a useful basis for starting preparation for the course. A few good textbooks and online resources such as the MIT open courseware page are useful for instructors putting together courses and are given in the supporting information.
Students are encouraged to look up references and presentation topics given in class using primary literature including at pubs.acs.org and sciencedi rect.com (both resources available at many smaller institutions). Industrial and government trade magazines such as Tech Briefs, Chemistry World, and Chemical and Engineering News (C&EN) were used to find exciting new applications of polymers and for helping students to design their inclass presentations. Other resources such as the Michigan State University polymer page (https://www2. chemistry.msu.edu/faculty/reusch/ VirtTxtJml/polymers.htm), UC Davis ChemWiki (http://chem.libretexts. org), and the book Polymers: Chemistry and Physics of Modern Materials (Cowie & Arrighi, 2008) were also used to reinforce student knowledge and develop lecture material.
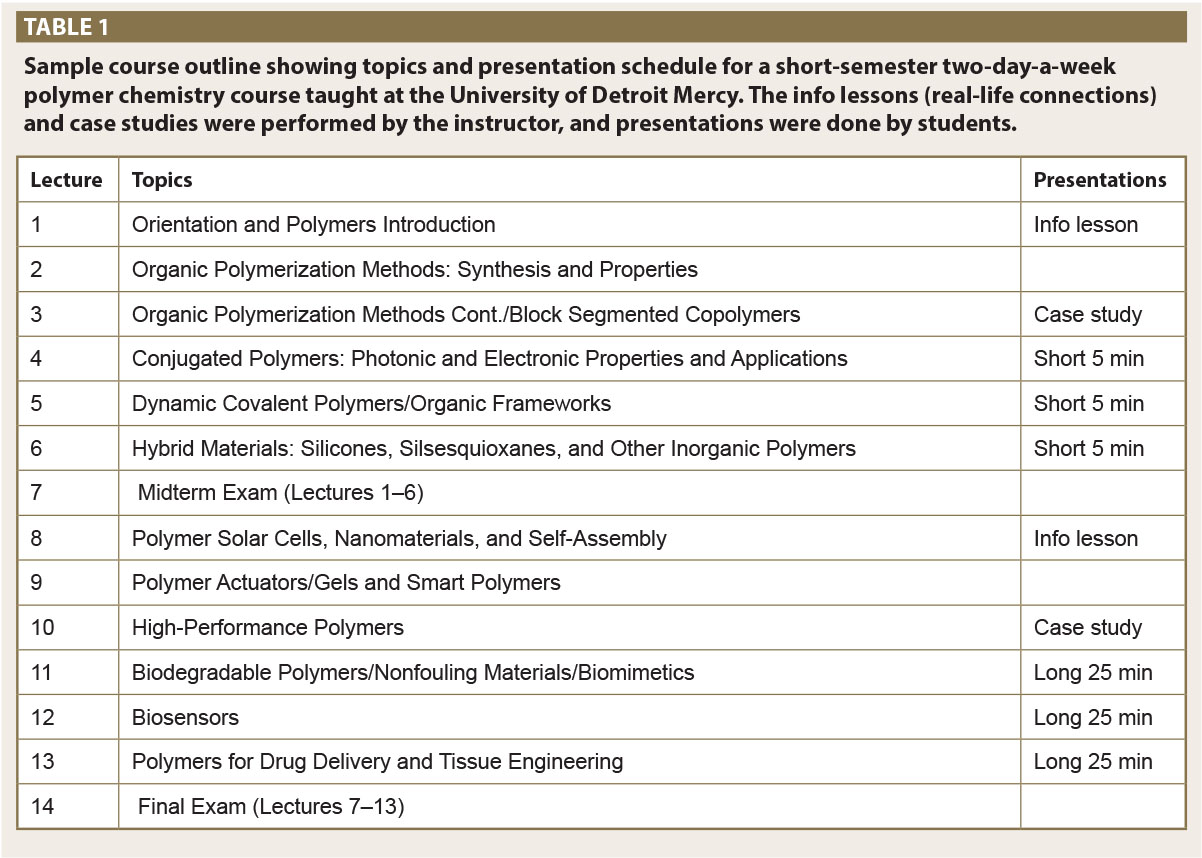
The course is split into two parts, which are roughly half a semester each (equivalent to 150 instructional minutes per week in a 15-week semester). The first part of the course goes through the basics of polymer chemistry including a brief history and discussion of Nobel prizes (Stahl, 1981), synthesis, and characterization, and the second half is mainly focused on the applications of interesting functional polymers (Table 1). Though the course is taught as a combination synthesis and applications, the two parts could easily be split into two separate courses covering in-depth knowledge of each topic area. Both parts are covered in this course to keep students excited for what “cool” applications are to come.
The course covers a wide range of topics including general polymerization techniques of basic polymer systems (i.e., polystyrene, nylon-6,6, etc.), block copolymers (structural motifs, types of blocks), conjugated polymers and their photonic and electronic properties and applications, high performance polymers (smart polymers, actuators, gels), hybrid polymers (silicones, silsesquioxanes, and other inorganic polymers) and biopolymers (drug delivery, tissue engineering, biodegradable, nonfouling, and biomimetics). The synthesis; properties; and industrial, biomedical, and optoelectronic applications of all of these materials are discussed. Students learn design principles to achieve specific functions from polymers, synthetic methodology, structure–property relationships, and fabrication of devices from polymers.
The course is designed as an introduction to polymer chemistry with a minimum requirement of undergraduate organic chemistry being completed so some topics such as kinetics and thermodynamics were only discussed in their simplest of terms, but depending on the level of your students, a more in-depth study may be appropriate. The polymerization reactions discussed are broken down as simply as possible to fundamental organic chemistry with each mechanistic step being shown in detail. This made the class move at a slower pace to ensure suitable understanding so that students could retain this information after course completion and use it throughout their careers, something more important than covering every subject in extreme detail in an introductory class, even without a laboratory component. Though a laboratory course in polymer chemistry is not presented here, it can be very beneficial to aid in gaining hands-on experience with many of the techniques (http://www. pslc.ws/macrog/lab/) but is likely resource limited at many schools. It is recommended for simplicity to include a polymerization experiment in the organic chemistry lab without the need for a full course; as an example, Matyjaszewski and others have designed simple procedures such as for controlled radical polymerization experiments (Beers, Woodworth, & Matyjaszewski, 2001; Tsarevsky, Woodruff, & Wisian-Neilson, 2016; Wackerly & Dunne, 2017).
If an instructor is interested in tying some aspects of this course into an organic chemistry course, some parallels can be made (Goh, 2013; Schaller, Graham, Jakubowski, & Johnson, 2017). As an example, condensation reactions are discussed in great detail in organic chemistry courses. Many polymers (i.e., epoxies, polyesters) are formed by condensation reactions as well. These could be easily integrated into those lesson plans and discussed as doing the same condensation reaction repeatedly to make a long chain.
Teaching methodology
Teaching is conducted through a variety of methods and is adjusted depending on the type of material being presented. Polymer synthesis methods are taught by board lecture, real-life examples, class discussion, and interactive case studies. This is a slower approach, but it often helps students solidify knowledge, have time to prepare to ask questions, and have better discussions. This slower pace method is especially important for students with very little or no exposure to polymers, and many of whom have no experience with radical initiated reactions and mechanisms. This method also allows for drawing clear connections with organic chemistry.
For the application part of the class, lectures are taught using a mix of computer-aided presentations and videos from scientific research publications (Kraft, Rankin, & Arrighi, 2012). These were supplemented by emphasizing important points and connections on the board to solidify concepts. The presentation videos allowed for showing the complexities and awe-inspiring nature of modern materials in a way unattainable through drawing. Most of the applications portion of the course is put together using primary literature from journals, such as Macromolecules, Polymer Chemistry, Chemical Communications, JACS, Nature, and Science. This allows for some of the newest and most exciting topics in polymer science to be presented and connected to the synthesis techniques examined in the first part of the course. The biggest challenge with using primary literature is that many of the articles are very advanced to upper level undergraduate or first-year masters students, so great care must be given to clarify and connect the advanced concepts back to the basic polymer chemistry learned in the first part of the class.
The lectures are broken up by a series of class and/or group activities. The first type of activity is a case study discussion (Campbell, Powers, & Zheng, 2015). This is most often a recent article using a polymerization method discussed in class. The problem is presented with background and split by either showing how the researchers solved the problem or how they gave the problem without a solution, in both cases using their learned knowledge to see what kind of method they could come up with. As an example, students are presented with a problem related to polymer membranes in proton exchange devices. The structure of a proton exchange device is discussed as well as limitations with current technology. The standard polymer Nafion is introduced and its potential limitations are determined through class discussion. A potential replacement is then introduced from using phosphonic acid block co-polymers made by anionic polymerization (Perrin, Elomaa, & Jannasch, 2009). A learning example is introduced showing the importance of structure in anionic polymerization, with unsterically hindered monomers undergoing nucleophilic substitution at the phosphonate instead of undergoing anionic polymerization. By adding steric bulk to the initiating monomer, polymerization proceeded. The differences in properties between Nafion and this new material are then compared and contrasted.
The second activity is the discussion of real-life applications of polymers examined in class. The C&EN series “What’s That Stuff?” and Tech Briefs are both useful in designing short info lessons (<5 minutes) about objects familiar to the students (https://cen.acs.org/collections/wts. html; https://www.techbriefs.co). For example, the history and materials evolution of golf balls over the years are discussed, with students being highly interested in these subjects and realizing that polymers are everywhere (Gorss, 2005)—a connection they had not really made before.
Demonstrations are also done during class, which is important exposure given the limited ability PUIs have in offering polymer chemistry in a lab setting (Rodriguez, Mathias, Kroschwitz, & Carraher, 1978). These demonstrations were hands-on and in a small course of <10 students. Many may be done as minilabs with each student doing his or her own experiment, such as the simple Borax and Elmer’s glue “putty” experiment (https://www. stevespanglerscience.com/lab/experiments/glue-borax-gak/). Demonstrations include showing the students the synthesis and then discussing the chemistry taking place. Examples include synthesis of nylon-6,6, a twopart epoxy resin, a series of silicone elastomers, and the multiple polymer makeup of a golf ball core. Seeing the chemistry happen in front of them really helped the students make connections between the structures they saw and the physical material that resulted, helping to alleviate the lack of a dedicated lab component through active classroom learning.
In conclusion, the development ideology and teaching methods of a polymer chemistry and applications course suitable for small colleges and universities has been discussed. The approaches described would allow instructors with little or no experience in polymer chemistry to develop their own courses and/or incorporate polymer topics into their core courses. Polymers will continue to be an important part of everyday life for many years to come (Ritter, 2002), and therefore education about their syntheses, function, and properties will only become more important.
Acknowledgments
I thank Mark Benvenuto at University of Detroit Mercy (UDM) for giving me the opportunity to teach a special topics course of my choosing. I also thank my postdoc advisor Timothy Scott at University of Michigan (UM) for allowing me to take this opportunity and Matt Mio and Kendra Evans at UDM for fruitful discussions and help with running the class. Last, I thank Anne McNeil and Jinsang Kim at UM for inspiring many of the topics discussed throughout the course.
Supporting information
The supporting information includes additional resources for course material, demonstrations, rubrics for presentations and example exam questions (available at http://www.nsta.org/college/connections.aspx).


