Idea Bank
A Chemistry Puzzle to Be Solved
The Science Teacher—February 2020 (Volume 87, Issue 6)
By Barry Friedman

Where did the color in the left beaker (which contained baking soda) go?
Adding baking soda to vinegar is a well-known, frequently used reaction that illustrates gas formation. Both ingredients are readily available from the grocery store, and both can be safely discarded down the sink. In order to jazz up this demonstration, colored gummy bears can be put into the vinegar before the baking soda is added. The idea is that the evolved gas bubbles will lift the gummy bears, enhancing the visual effect of the gas formation. Since gummy bears are heavy and large compared to the size of the gas bubbles, the gummy bears can be sliced into small pieces before being put into the vinegar.
The suitable demonstration procedure is as follows:
- Cut 24 gummy bear candies (must be colored) with a knife or scissors into very small pieces.
- Fill two 600 ml beakers halfway with water.
- Put equal amounts of gummy bear pieces into each beaker.
- Pour 100 ml of vinegar into each beaker.
- Put approximately 1 tablespoon of baking soda into one of the beakers but not the other.
- Label the beakers to indicate their contents.
After a short amount of time, the gummy bear pieces in the beaker with the baking soda will start floating to the surface and then fall down in a cascade-like effect, due to carbon dioxide gas being produced by the reaction of vinegar and baking soda. As the carbon dioxide gas bubbles rise to the top, they bring the small gummy bear pieces with them. The beaker with no baking soda produces no carbon dioxide gas, and the gummy bear pieces just sit at the bottom.
One day I cheerfully performed this demonstration in front of my class and noticed that the results were unremarkable. After class ended, due to the limited amount of time before the arrival of students for the next class, I left the setup on the bench top until the following morning. At the end of the day, the contents of both beakers looked identical: little pieces of colorful gummy bears in clear liquid.
The following morning, however, when I approached the beakers to discard the contents and clean them, I noticed that the beaker that had the baking soda added to it contained completely clear liquid and no solid material. The other beaker still contained pieces of colorful gummy bears. I was surprised! What had taken place? Where did the gummy bear pieces go? Where did the color go?
The reaction of the acetic acid in the vinegar with sodium bicarbonate (i.e., baking soda) in solution produces carbon dioxide along with sodium acetate and water (see Figure 1).
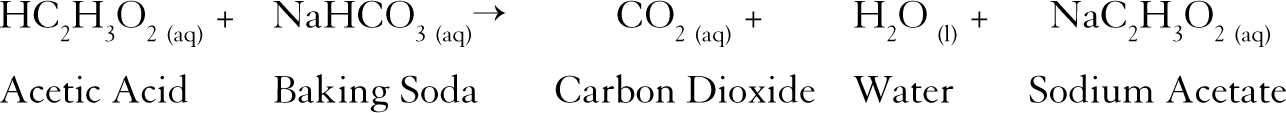
The reaction of the acetic acid in the vinegar with sodium bicarbonate (i.e., baking soda) in solution produces carbon dioxide along with sodium acetate and water.
The beaker with the added baking soda contained carbon dioxide produced during the reaction. The beaker without baking soda added produced no carbon dioxide. Could the presence of carbon dioxide cause the solid gummy bears to disappear? Could the presence of the carbon dioxide cause the color to disappear?
The traditional gummy bear is made from a mixture of cornstarch, beeswax, carnauba wax, citric acid, starch, fractionated coconut oil, sugar, dextrose, corn syrup, natural and artificial fruit flavors, glucose syrup, and artificial colors (yellow 5, red 40, and blue 1). The food dye molecules in the artificial colors have large electron clouds because of highly extended conjugated π bonding.
If carbon dioxide were an oxidizing agent, a reasonable scenario can be formulated. The generated carbon dioxide causes oxidative cleavage of the polyene of the food dye. The π-electron cloud of a double bond, located above and below the molecular plane, is polarizable. This can lead to the process in which the carbon–carbon bonds are broken and the initial alkene carbons are oxidized to form carbonyl compounds. This is analogous to oxidative cleavage of alkenes involving the permanganate anion, the periodate anion, and ozone.
Under this assumption, the generated carbon dioxide reacts with the food dye molecules via a redox reaction to break them into fragments. This fragmentation eliminates the color (see Figure 2)
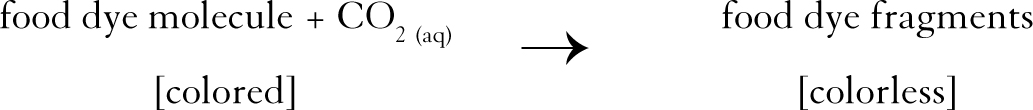
Generated carbon dioxide reacts with the food dye molecules via a redox reaction to break them into fragments.


