A Learning Tool for Chemistry and Health Professions Students: Mnemonics for Writing Net Ionic Equations
Journal of College Science Teaching—January/February 2020 (Volume 49, Issue 3)
By Angela L. Mahaffey
Chemical mnemonic devices have been designed to aid students in understanding chemical concepts in previous years. This has been done for concepts such as oxyanions, ozonolysis, tautomerization mechanisms in organic chemistry, and writing reactions of metals with nitric acid. One chemical concept introduced to students in the chemistry and nonchemistry degree programs is solubility and chemical element group chemistry reactions. A main component of group chemistry is precipitation reactions and net ionic equations. Three separate groups of health professions students, totaling 136 students, were surveyed using Sakai (an open-source CLE software) on the difficulty of concepts within physical science courses taken in accordance with their program’s core curriculum. The results of this student poll illustrate that undergraduate health professions students (nursing, medical sciences, exercise sciences, etc.) perceive chemistry courses as presenting the most difficult concepts, for all three groups. A separate survey was also performed, asking students the year of their last high school chemistry course. The majority of health profession students polled that their second year of high school was their last year of high school chemistry. This highlighted a need for learning aides: two useful chemical mnemonic devices have been designed to aid students in writing net ionic equations.
Chemistry textbooks for both health professions and chemistry students are comprised of book sections describing aqueous reactions, solubility products, group chemistry, and precipitation reactions. Previously, a few mnemonic devices have been successfully designed to help students understand chemical concepts (DeLoach, 1960; Hawkes, 1990; Kurushkin, 2015; Ruekberg, 2011; Stephens, 2010). There is little to no doubt that introductory chemistry courses integrate concepts surrounding group chemistry and precipitation reactions in course content. On the topic of reactions in aqueous solutions, precipitation reactions abound. These reactions yield solid products from aqueous solutions in which the reactants of homogenous solutions interact to form a compound in a heterogeneous mixture. Understanding the solubility properties of aqueous (aq) chemical compounds and how these compounds react to produce a solid product is an important part of the introductory chemical education process. Such reactions can yield products in the liquid (l), solid (s) and gaseous (g) states. Tracking the interactions of the anionic and cationic chemical species as they produce the solid product is a task best completed in writing net ionic equations (Brown et al., 2018; Hawkes, 1990; Raymond, 2014).
Two mnemonics for writing net ionic equations
An anonymous and voluntary digital poll of 136 health professions students was performed to survey which of several core curriculum science courses included the most difficult concepts (Table 1). Chemistry was selected as most difficult by the majority of students polled. To ensure students were registered in the health professions programs, a collaboration and learning environment (CLE) software (Sakai) registered to the university was employed and the anonymous and voluntary poll was constructed (Apereo Foundation, 2018). Students were separated into three groups, and each group completed the digital poll separately. As chemistry for health professions courses review reactions in aqueous solutions, concepts such as precipitation reactions and net ionic equations are also introduced in the curriculum. One struggle for students in the health professions (or other nonchemistry programs) is transcribing a complete net ionic equation from an unbalanced molecular equation. Granted, the concept may have been introduced in high school textbooks (Sarquis & Sarquis, 2012), but student recall may present an issue. Health professions students also completed a digital poll, where they were asked to select the year in high school in which they were last enrolled in a chemistry course (Figure 1). On completion of this anonymous and voluntary survey of 130 students, only 15 students noted having completed a chemistry course their fourth year of high school. These are shocking results. So, here are two mnemonic devices designed to help students recall steps for correctly writing a net ionic equation: (a) “Take a MINute.” and (b) “Don’t touch the LeGS!” These chemical mnemonic devices may be helpful for future undergraduate physical sciences and health professions students enrolled in introductory chemistry courses.
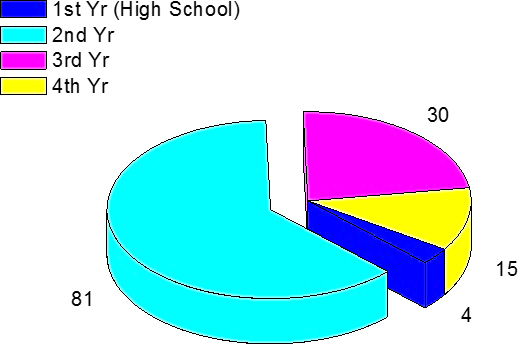
Using a CLE software, 130 students were surveyed via an anonymous and voluntary digital poll (Sakai) (Apereo, Foundation 2018), ensuring students were registered in health professions programs. In this poll students were asked the following: “What year in high school did you last take a chemistry course?” For 81 of the students polled, the answer to this question was the second year of high school. The poll results were graphed using Origin (OriginLab, Northampton, MA).
Using a CLE software, 130 students were surveyed via an anonymous and voluntary digital poll (Sakai) (Apereo, Foundation 2018), ensuring students were registered in health professions programs. In this poll students were asked the following: “What year in high school did you last take a chemistry course?” For 81 of the students polled, the answer to this question was the second year of high school. The poll results were graphed using Origin (OriginLab, Northampton, MA).
| Table 1 | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||
“Take a MINute” and “Don’t Touch the LeGS!”
The mnemonic “Take a MINute” can remind students to pause and fully assess the step-by-step process for writing a net ionic equation for a given precipitation reaction. Additionally, the chronological measurement, minute, is often abbreviated as “min.” This abbreviation can help explain the three steps for writing a net ionic equation: M-I-N, as in Molecular equation, Ionic equation and Net ionic equation (Figure 2, p. 29). Here we demonstrate how to write a net ionic equation for the precipitation reaction of iron(III) bromide and potassium phosphate to produce the precipitant iron(III) phosphate (Figure 3, p. 30; Brown et al., 2018). Using the “Take a MINute” mnemonic device (Equations 1–3, Figure 2a–c), we balance the molecular equation for the reaction between iron(III) bromide and potassium phosphate (M; Equation 1). For mnemonic step “M,” students are provided with an analogy referring to the reactants as anionic/cationic “dance partners” and then instructed to switch the anionic “dance partners” of the cation reactant compounds similar to a form of “square dancing”; this procedure assisted students in predicting the products. Further, students are provided with the following resources, which can be located in a number of general chemistry textbooks (Brown et al., 2018; Hawkes, 1990; Raymond, 2014): (1) A list of common cations (i.e., Groups 1, 2 and transition metals) such as Potassium (K+), Calcium (Ca2+), and Iron (II)/Iron(III) (Fe2+/Fe3+)—and polyatomic cations such as ammonium (NH4+). (2) A list of common polyatomic anions such as Hydroxide (OH-), Nitrate (NO3-), Sulfate (SO42-),Carbonate (CO32-), Phosphate (PO43-)—and halides (such as Chlorides; Cl-, Bromides; Br- and Iodides; I-). Last, students have access to a list (or table) of solubility rules noting: (1) ALL Nitrate (NO3-) compounds are water soluble and thus aqueous (aq). (2) Halide compounds (containing Chlorides; Cl-, Bromides; Br- and Iodides; I-) are soluble with the exception of Lead (Pb2+), Silver (Ag+), and mercury (Hg22+). (3) Sulfate (SO42-) compounds have similar solubility exceptions as halide compounds with Strontium (Sr2+), Barium (Ba2+), Lead (Pb2+), and Mercury (Hg22+) sulfate compounds being water insoluble (s; solid phase compounds). (4) Regarding water insolubility rules (s; solid phase compounds), a list including hydroxides (OH-) as being solid (insoluble) with the exceptions of Ca2+, Ba2+ and Sr2+ (aqueous hydroxide compounds). For Phosphates (PO43-) and Carbonates (CO32-), the water insoluble products (s) would be all phosphate compounds excluding those with Ammonium (NH4+) as a cation (Brown et al., 2018; Hawkes, 1990; Raymond, 2014). Metaphorically “armed” with the preceding details, students are instructed to identify the cation and anion “square dancing partners” of the reactants when predicting the molecular equation. For example, using Equation 1 (Figure 3, p. 30), the cation of the first reactant iron(III) bromide (FeBr3) is the iron metal cation (Fe3+) and halide anion “partners” are three bromide ions (3Br-); for the second reactant, the metal cations are three potassium (3K+) cations “partnered” with the polyatomic anion phosphate (PO43-) “dancer.” Here, students are instructed to “switch” the anion “dance partners” (Br- and PO43-) of the cation “dancers” (Fe3+ and K+) for the two reactants (FeBr3 and K3PO4)—in a method coined here as “square dancing (cation/anion) reactants to predict the new products.” Finally, students are encouraged to keep tally of all “dancers.” To elaborate, in Equation 1 (Figure 3,, p. 30), there are three bromide (3Br-) anions “dancing” with the iron (III) Fe3+ metal cation, on the reactant side of the equation. Students are informed that the three positive charges (+3) of the iron (III) Fe3+ metal cation can “pair” with each of the three negative charges (–3) of the three bromides (3Br-), causing Fe3+ and 3Br- to be acceptable “dance partners.” So, now there should be three bromide ions (3Br-) “dancing” with their new cation “partners” (three positive potassium ions; 3K+) once the “dance partners” are switched, which leaves the Fe3+ cation “dancing” (or “partnered”) with the PO43- anion. To assist student understanding, the resulting iron(III) phosphate pairing can be explained similarly as the potassium ions (3K+) and bromide ions (3Br-) pairing. Considering molecules are dynamic, the term “dancing” is not a far-fetched analogy (a humorous note). Hence, through tallying the number of metals and nonmetals on either side of the chemical equation and tracking these ionic charges, students are able to balance the molecular equation using the “square dancing (cation/anion) reactants to predict the new products” analogy. Regarding the phases of the new products, at this final step in writing the molecular equation, students refer to the solubility rules in their textbook, which note the iron(III) phosphate (FePO4) product is water insoluble (s; solid), and the potassium bromides (3KBr) are water soluble (aq; aqueous). Next, for the ionic portion of the “MIN” mnemonic, we separate compounds into ionic (I; Equation 2) counterparts using the second mnemonic (see “Don’t touch the LeGS!” mnemonic). This helps us to identify the bromide and potassium spectator ions and write our finalized net ionic equation (N; Equation 3). See the three equations in Figure 3.
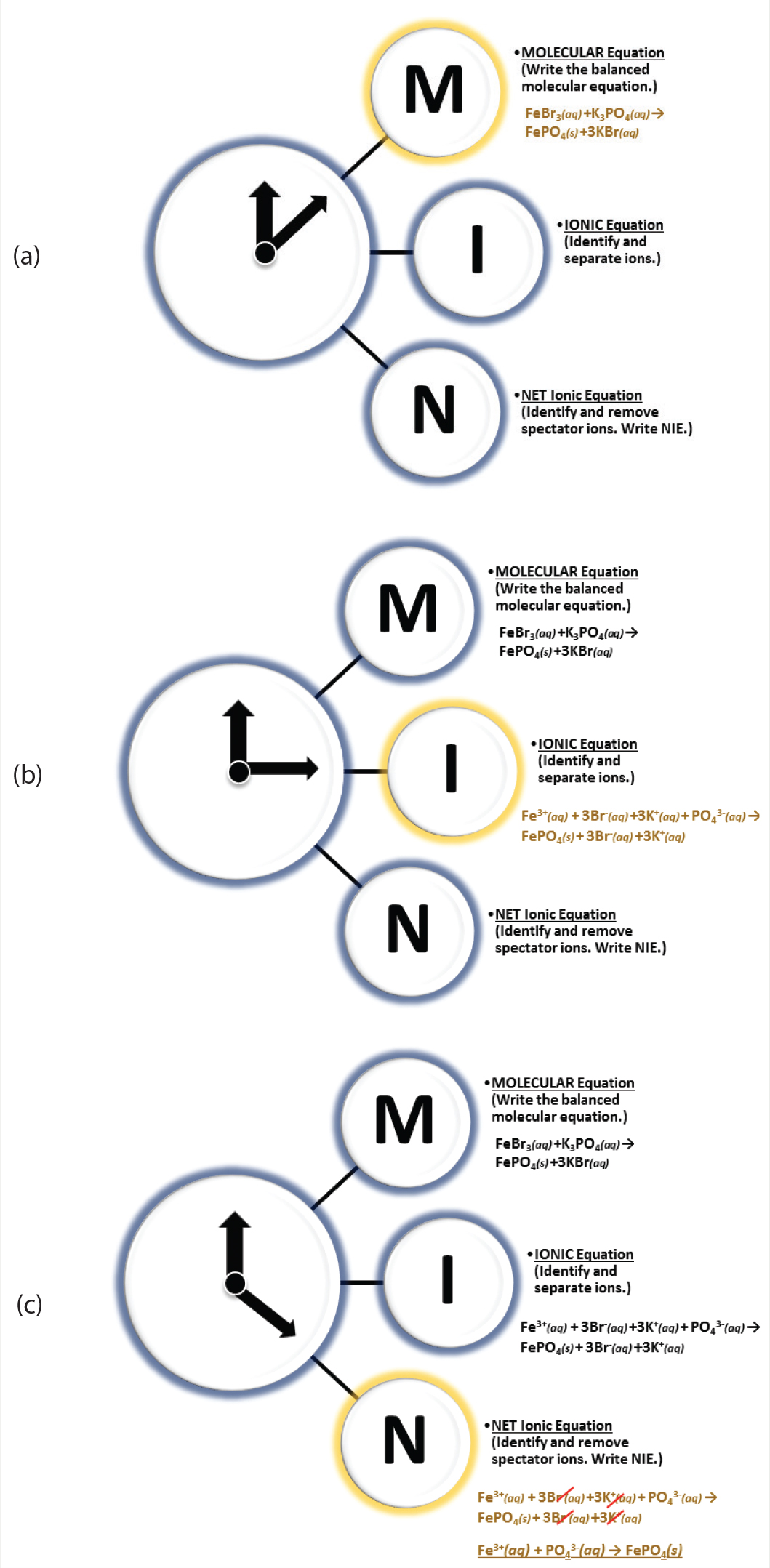
Diagrams of the “Take a MINute” and “Don’t touch the LeGS!” chemical mnemonic devices. (a) Illustrates the use of “M” of the mnemonic device (writing a balance MOLECULAR equation), (b) Shows “I” of the mnemonic device, providing students with a reminder to write the IONIC equation for the balance molecular equation by separating aqueous reactants and products into ionic counterparts and (c) The highlighted “N” help students to recall the final step of writing a net ionic equation by removing spectator ions and transcribing the final (NET IONIC) equation with remaining reactants and products.
Diagrams of the “Take a MINute” and “Don’t touch the LeGS!” chemical mnemonic devices. (a) Illustrates the use of “M” of the mnemonic device (writing a balance MOLECULAR equation), (b) Shows “I” of the mnemonic device, providing students with a reminder to write the IONIC equation for the balance molecular equation by separating aqueous reactants and products into ionic counterparts and (c) The highlighted “N” help students to recall the final step of writing a net ionic equation by removing spectator ions and transcribing the final (NET IONIC) equation with remaining reactants and products.
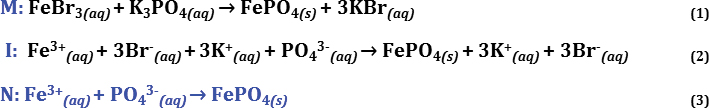
Precipitation reaction of iron (III) bromide and potassium phosphate: an example net ionic equation.
Precipitation reaction of iron (III) bromide and potassium phosphate: an example net ionic equation.
“Don’t Touch the LeGS!”
Another mnemonic device is “Don’t touch the LeGS!” As there are four states of matter—as reviewed in GOB (general, organic, and biological chemistry) textbooks—in which reactants and products can exist (liquid, solid, gas and aqueous), this helps to remind students to focus on the aqueous compounds in writing a net ionic equation. During the “I” (ionic equation) step that requires compounds to be separated into cations and anions, this mnemonic can serve to remind students that the liquid (l), gaseous (g), or solid (s) compounds (l-g-s; “legs”) cannot be separated into anions or cations—only aqueous (aq) phase reactions. So, “Don’t touch the legs!” (do not separate the (l), (g), (s) state compounds into ions), and with the help of this mnemonic, students are reminded on how to successfully complete the net ionic equation step as seen in Equation 2 (writing ionic equation; Figure 2b).
Conclusion
To sum, it is notable that a percentage of solubility laws and group chemistry, aqueous reactions, and precipitation reactions concepts (which includes net ionic equations) are topics in select high school textbooks (Sarquis & Sarquis 2012; Figure 1). Notwithstanding, let us consider the addition of the two mnemonics provided in this article can serve to help undergraduate students recall how to apply the rules of writing net ionic equations. These mnemonics were incorporated into dozens of lecture and laboratory class content, which includes hundreds of undergraduate (health professions and physical science major) students. As a supplement to precipitation reaction general chemistry course content, students noted the ease of use and proficiency in recall of the net ionic equation rules, when employing these rote learning tools: two mnemonics. So remember, when writing net ionic equations students can recite the following: “Take a MINute” and “Don’t touch the LeGS!”
Angela L. Mahaffey (amahaf1@luc.edu) is an Assistant Professor in the Marcella Neihoff School of Nursing at Loyola University Chicago.
Chemistry Teaching Strategies Postsecondary


