Research & Teaching
Adaptation of Project-Based Learning Concepts to the Organic Chemistry I Laboratory Curriculum in a Small College Environment
Journal of College Science Teaching—March/April 2023 (Volume 52, Issue 4)
By Ilirian Dhimitruka and Geetha Surendran
Project-based learning (PBL) research is shown to improve students’ higher-order thinking skills. In this article, we describe an adaptation of the PBL model that is suitable for colleges with limited science, technology, engineering, and mathematics research capabilities. Our objective was to design a meaningful research project requiring minimal extracurricular resources. A PBL sequence of 4 weeks, centered around three experiments, was embedded into the traditional curriculum. Students were asked to identify ethanol-water combinations that yield basil plant extracts rich in hydroxycinnamic acids, with optimal ultraviolet screening capabilities. The project addressed an issue of health and environmental concern that students found appealing. Instructors provided handouts, literature search guidelines, and training. Individual students solved part of the PBL project; to achieve meaningful conclusions, students were compelled to collaborate. The learning goals were to gain experience in performing an efficient literature search, performing mathematical calculations, plotting informative and aesthetic graphs, identifying relations between theoretical concepts and experimental data, and presenting conclusions to a scientific audience. Feedback was obtained using a Student Assessment of their Learning Gains instrument. Students presented their research in local conferences. The PBL sequence concept, supplementing the traditional curriculum rather than replacing it, proved to be a successful active and inclusive teaching practice.
Participation in experimental research is shown to improve students’ overall performance in science, technology, engineering, and mathematics (STEM; Hofstein & Lunetta, 2004). However, extracurricular research opportunities are limited to few high-achieving students. Opportunities are not available for the majority of students who would benefit academically. Course-based undergraduate research experiences (CURE) and project-based learning (PBL) are semester-long activities that substitute “cookbook-style” curricula with real-life research projects (Kovacevic et al., 2020). CURE and PBL models allow students to experience the scientific method, formulate hypotheses, design experiments to test hypotheses, and analyze data to formulate theoretical models (Ram, 1999). Besides learning laboratory skills, students participating in CURE and PBL research develop a better understanding of underlying theoretical concepts. For example, the connection between macroscopic properties, molecular and submicroscopic properties, and chemical symbols is one of the main challenges of teaching chemistry, a challenge that CURE and PBL projects address efficiently compared with traditional teaching practices (Johnstone, 2000).
Typically, CURE or PBL projects are performed in research-intensive universities with large cohorts of students majoring in STEM subjects (Clark et al., 2016; Cruz et al., 2020; Slade et al., 2014). Statistical instruments are implemented in large cohorts that allow students and instructors to self-evaluate accurately the impact of new practices for improving student attitude toward STEM subjects (Burrows et al., 2017; Corwin et al., 2015; Shortlidge & Brownell, 2016). Research-intensive universities have the benefit of ample financial resources, instrumental facilities, and teaching assistants, which are necessary to ensure the success of CURE or PBL research despite students’ inexperience (Cascella & Jez, 2018). Adapting CURE or PBL models to a small, urban college environment presents unique challenges. Our financial and logistical resources are limited. The number of students who can participate in such practices is small, which makes the statistical assessment of learning goals difficult. A large part of our student population is composed of nontraditional students majoring in professional health sciences who are less likely to spend the extra time needed to succeed in research projects.
To address these challenges, we envisioned the use of a short PBL sequence embedded into the normal curriculum. By definition, a PBL experience addresses an authentic research inquiry of significant value, using established literature methods. Our main goal was to offer our students the benefits of experiencing a real-life research project without forcing changes to the curriculum structure. Our learning objectives were to encourage students early in their studies to improve their logical reasoning, analytical, mathematical, computer, and presentation skills. Additionally, we hoped to inspire more of our students to participate in extracurricular research opportunities. The ideas we present in this article could be adapted to other science programs besides organic chemistry.
The PBL sequence was implemented over 4 weeks at the end of the semester-long Organic Chemistry I laboratory. This class covers the knowledge and skills necessary to synthesize, isolate, purify, and characterize organic compounds. In particular, we perform laboratory experiments on boiling and melting point, solubility, density, refractive index, steam and normal distillation, recrystallization, liquid-liquid extraction, solid-liquid extraction, and thin layer chromatography (TLC), as well as a theoretical workshop on spectroscopic methods. Prior to the start of the PBL sequence, we maintained the same curriculum structure for the first 11 weeks of the semester. Students became proficient in all of the basic laboratory techniques. The PBL sequence incorporated experiments on solid-liquid extraction, TLC analysis, and ultraviolet (UV) spectroscopy, topics covered in the traditional curriculum.
The title of the PBL sequence was UV Spectroscopy Analysis of the Potential of Plant Extracts Enriched in Hydroxycinnamic Acids (HCAs) to Offer Sunscreen Protection. The long-term aim of our research is to identify UV filters from natural sources to replace commercial UV filters in sunscreens, which are suspected of adverse effects on human health and marine environments (Ekpeghere et al., 2016; Matta et al., 2019; Schneider & Lim, 2019). In a recent study, we estimated via UV spectroscopy that HCAs exhibit broadband UV screening properties comparable to or better than commercial UV filters (Surendran et al., 2019). We chose dry basil leaves as our model plant because they are known to contain substantial amounts of HCAs. Students were asked to determine, by collaborating with one another, the optimal ethanol-water ratio that produces a basil extract rich in HCAs with suitable UV screening properties.
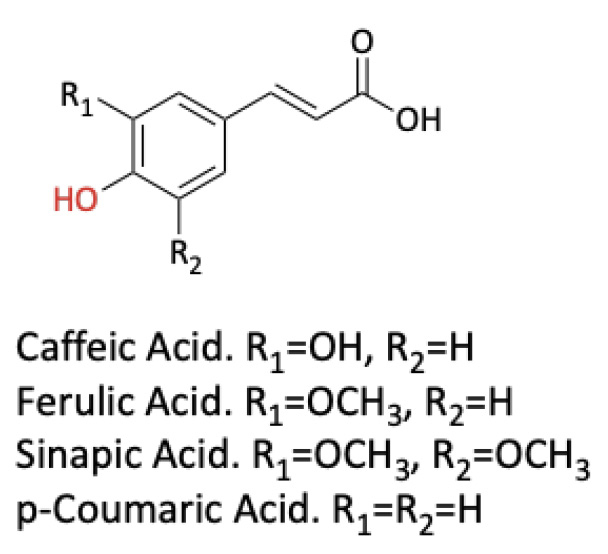
Structures of hydroxycinnamic acids.
Design of the PBL sequence
The PBL sequence was completed over four sessions of 3 hours per week. Overall, 15 and 18 students participated in the spring and fall 2019 semesters, respectively. Students were enrolled in biology or health science programs. All students had completed General Chemistry I and II lectures and corresponding laboratory requirements. Students worked in groups of two. Two groups were assigned one ethanol-water solvent ratio to study. Data were collected independently by instructors to ensure the accuracy of the results, for a total of three measurements per data point.
Learning goals of the PBL sequence
Besides helping students acquire skills taught via expository experiments of solid-liquid extraction and TLC analysis, we addressed the following learning goals:
- Become proficient in the use of online search engines of scientific literature.
- Become proficient in the use of UV spectroscopy, Beer-Lambert law, and calibration curves to determine the concentration of a substance in solution.
- Work collectively to compare research data, draw conclusions, justify original hypotheses, and formulate theoretical explanations.
- Become proficient in the use of editorial tools to present research data, specifically Microsoft Excel, Word, and PowerPoint.
Experimental procedures
Chemicals were purchased from commercial suppliers and used without further purification. Thermo Scientific and Mettler Toledo UV5 spectrophotometers were used to collect UV-Vis spectral data. Dry basil leaves were purchased from local stores. Calculations were performed using Excel. This was a green project that posed minimal chemical hazard. Standard safety rules for working in a chemical laboratory and disposing of chemicals were applied.
Week 1: Solid-liquid extraction
We mixed 2 grams of finely chopped dry basil leaves with 70 milliliters of an ethanol-water solution in a 250 milliliter round-bottom flask. The mixture was stirred vigorously for 90 minutes at room temperature. The basil extract was filtered under vacuum, brought to 100 milliliters in a volumetric flask, and saved for later use. Students observed the purity of their extracts via TLC analysis using chloroform-methanol and hexanes-ethyl acetate as eluent.
Week 2: UV spectroscopy analysis of HCAs content
The UV spectra of basil extracts were recorded at various dilutions to ensure the linearity of measurements. Students estimated the concentration of HCAs in basil extracts using the Beer-Lambert law, A = εcl, using an average ε = 18,000M-1cm-1 at λ=325nm for the mixture of HCAs. Average amounts of HCAs in dry basil weight were estimated from their concentrations (Table 1).
Week 3: Analysis of the antioxidative capacity of basil extracts
Antioxidative capacity was measured using the 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay. Students recorded the decay of the absorbance of DPPH radical at λmax = 517 nm over time, upon mixing a solution of DPPH radical in ethanol with an appropriate dilution of basil extract, which contains approximately equimolar quantities of HCAs (Brand-Williams et al., 1995; Foti et al., 2004).
Week 4: Final day of class
Students submitted two lab reports during the course of the PBL sequence. Each group prepared a final PowerPoint presentation that included an overview of the literature, a summary of experimental data, and concluding remarks.
Data analysis
Sun protection factor (SPF) is a measure of the sunscreen’s ability to protect skin from sunburn (erythema), upon exposure to UVB rays (290–320 nm). The in vivo SPF is defined as the ratio between the amount of radiation needed to cause minimal persistent erythema (MED) with and without sunscreen (Osterwalder & Herzog, 2009). We used, for comparative purposes, in vitro SPF values, which were estimated from UV spectra according to the Mansur equation (Dutra et al., 2004; Sayre et al., 1979).
The ratio of UVA to UVB and critical wavelength λc was calculated from UV spectra to evaluate the potential of basil extracts to offer broadband protection from UVA (320–400 nm) and UVB (290–320 nm) solar radiation (Diffey, 2009). A ratio of UVA to UVB of greater than one is indicative of significant broadband protection. The critical wavelength λc was calculated as the wavelength at which the integral of spectral absorbance curve reaches 90 percent of the integral over the UV spectrum from 290 to 400 nm (Diffey, 1994). A critical wavelength above 370 nm indicates excellent broadband protection in the 290-400 nm range of the solar radiation (Diffey, 1994).
Results and discussion of experimental data
The objective of this PBL sequence was for students to determine, by working collaboratively, the optimal ethanol-water ratio that produces a basil extract rich in HCAs. Each group of students was assigned one volumetric ethanol-water ratio to study. The activity was engaging and inclusive. Students had to communicate with and help one another and interact with instructors to achieve reasonable conclusions.
Table 1 reports the collection of data obtained by students. The highest amount of HCAs, 23.03 mg/2 g of dry basil, was extracted using a 40% ethanol-in-water solution. Extracts obtained with absolute ethanol contained very low concentration of HCAs and showed significant absorbance above 400 nm in the visible region, consistent with the presence of flavanols and anthocyanins. As the water content was increased in the ethanol-water extracting solution, the UV absorbance of basil extracts shifted below 400 nm (Figure 2).
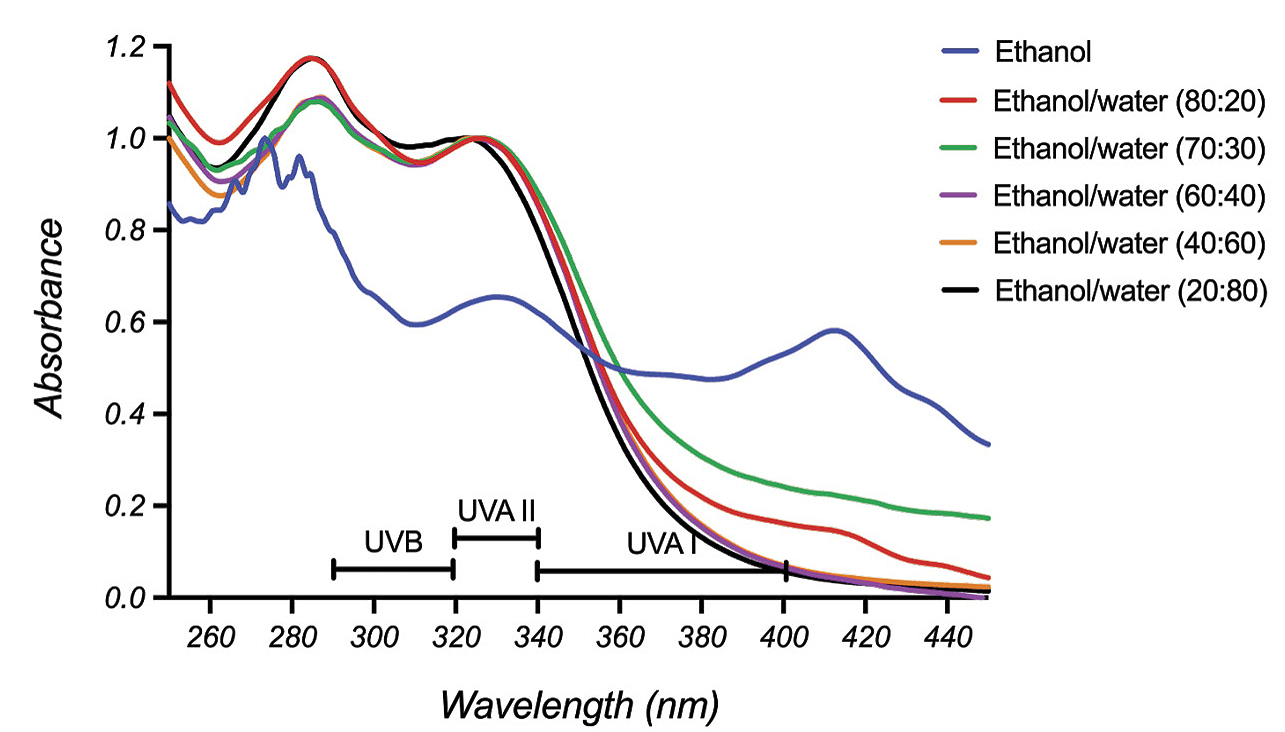
UV spectra of basil extracts.
Note the absence of absorbance above 400 nm in the UV-Vis spectrum of the basil extract obtained with 70% ethanol in water (Figure 3). Similar UV-Vis spectral profiles were observed for all ethanol-water basil extracts. TLC analysis confirmed UV-Vis spectral results. Colored fractions were observed in the TLC plates developed from basil extracts obtained with pure ethanol.
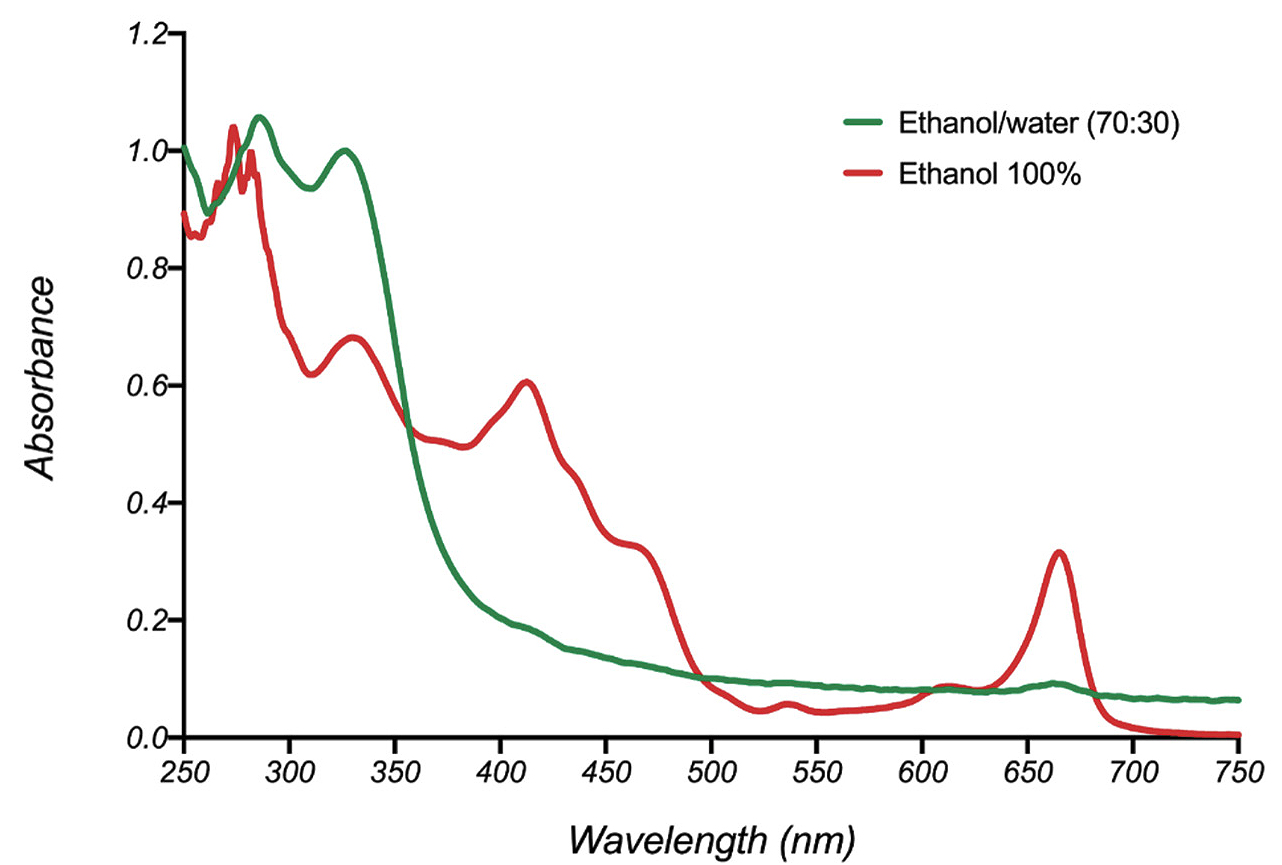
UV-Vis spectra of basil extracts.
The 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay is used routinely to confirm the presence of phenolic antioxidants such as HCAs in plant extracts (Foti et al., 2004). Alcoholic solutions of DPPH radically absorb visible light at λmax = 517 nm. The assay monitors the decay of the absorbance signal at λ = 517 nm, which is accompanied by a change of color from deep purple to yellow, upon scavenging of DPPH radical by antioxidants (Figure 4). The assay is reported as the percentage of the remaining DPPH radical over time (Brand-Williams et al., 1995).
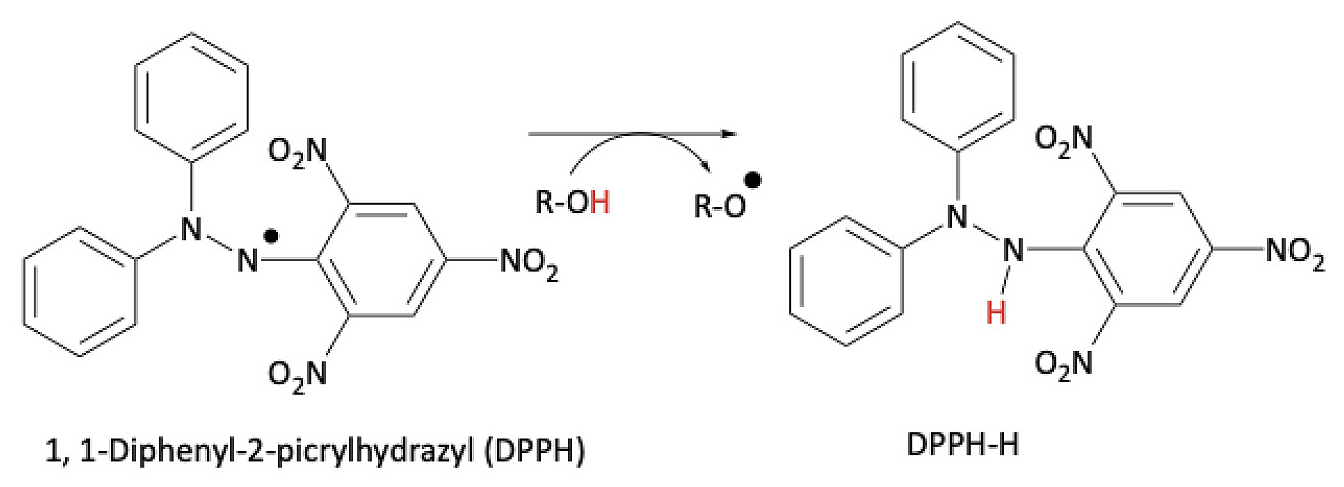
DPPH radical scavenging assay.
Students reported the reduction of DPPH radical in agreement with calculated concentrations of HCAs in their respective basil extracts. One representative example is shown in Figure 5. According to the literature, 1 mol of caffeic acid reacts stoichiometrically with 4 mol DPPH, 1 mol of sinapic acid reacts with 1.3 mol DPPH, and 1 mol of ferulic acid reacts with 2 mols DPPH (Brand-Williams et al., 1995). Kinetically, HCAs are reported to react quickly with DPPH radical, with 80% of the stoichiometric amount consumed within the first minute (Foti et al., 2004). Results supported the presence of HCAs in basil extracts, particularly the presence of caffeic acid and sinapic acid (Figure 5).
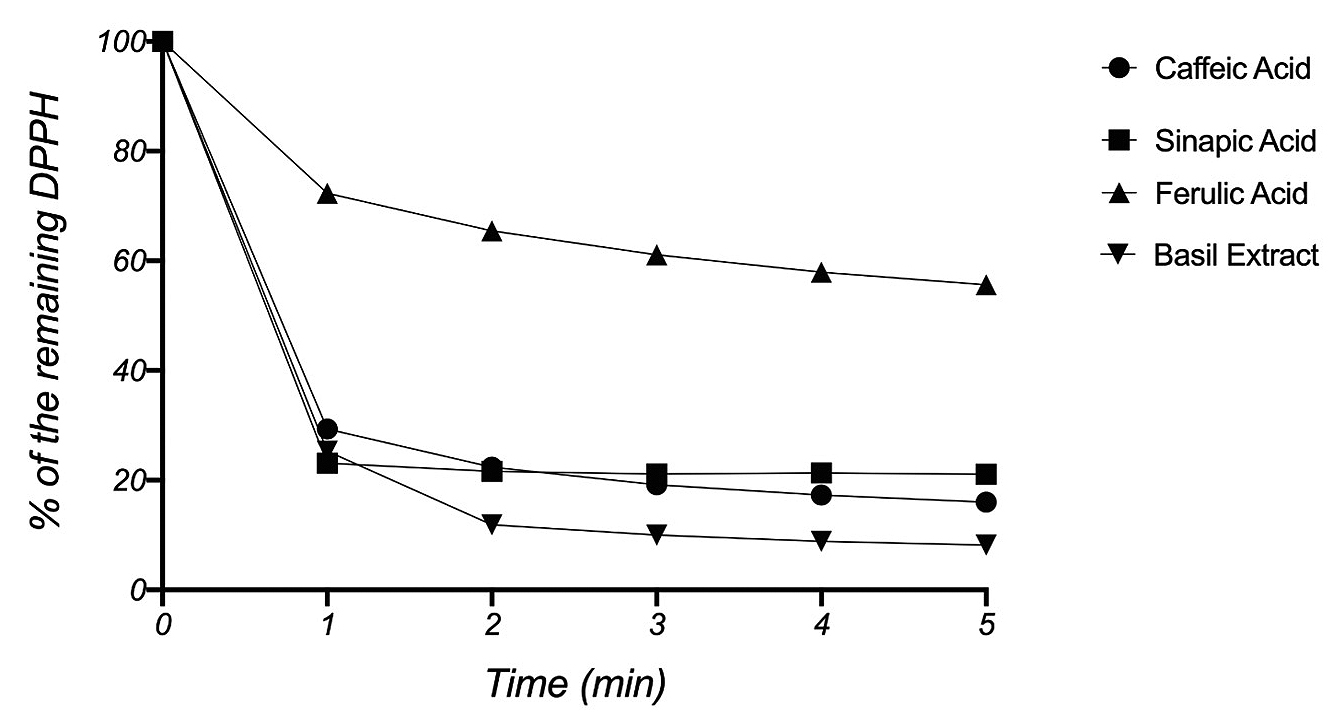
Analysis of antioxidative properties via DPPH assay.
Note. Concentrations of ethanolic solutions: [DPPH]/[basil] = 70 μM/20 μM, [DPPH]/[CA] = 74 μM/15 μM, [DPPH]/[SA] = 84 μM/60 μM, [DPPH]/[FA] = 79 μM/60 μM.
SPF values were calculated from the UV spectra of basil extracts. The amount of HCAs extracted from 2 grams of dry basil was assumed added to 1 gram of a hypothetical sunscreen and was counted as percent UV filter. In addition, critical wavelength λc and UVA/UVB values were calculated from the UV spectra of basil extracts. They are intensive properties and indicators of broadband UVA-UVB protection. Sun protection parameters of basil extracts compared well with values calculated from the UV spectra of two commercial broadband sunscreens, relative to their percent UV filter content (Table 1). Values of UVA/UVB = 1.56 and λc = 371 nm for the 70% ethanol-water basil extract were almost identical with those of commercial sunscreens. The highest concentration of HCAs was measured for the 40% ethanol in water basil extract; however, a slightly lower λc = 361 nm and UVA/UVB = 1.32 was observed.
One possible reason for the variation in λc is the presence or absence of extracted flavanols such as quercetin, which are soluble in ethanol. Flavanols absorb UV light at λmax = 390 nm. The critical wavelength λc of basil extracts decreased as the ethanol content was decreased and the water content was increased in the extracting solvent. This observation is consistent with the presence of a higher concentration of HCAs and a lower concentration of flavanols. The addition of water in ethanol was necessary to ensure HCAs were extracted preferentially, compared with metabolites that absorb in the visible range (Figure 3). As a result, the UV spectra of basil extracts obtained with ethanol-water mixtures exhibit a similar profile to the UV spectra of commercial broadband sunscreens (Figure 6). We concluded that basil extracts obtained with 40–70% ethanol in water would be suitable for use as additives with broadband UV screening properties, due to a combination of high concentration of HCAs, UVA/UVB >1, and λc ∼ 370 nm (Table 1).
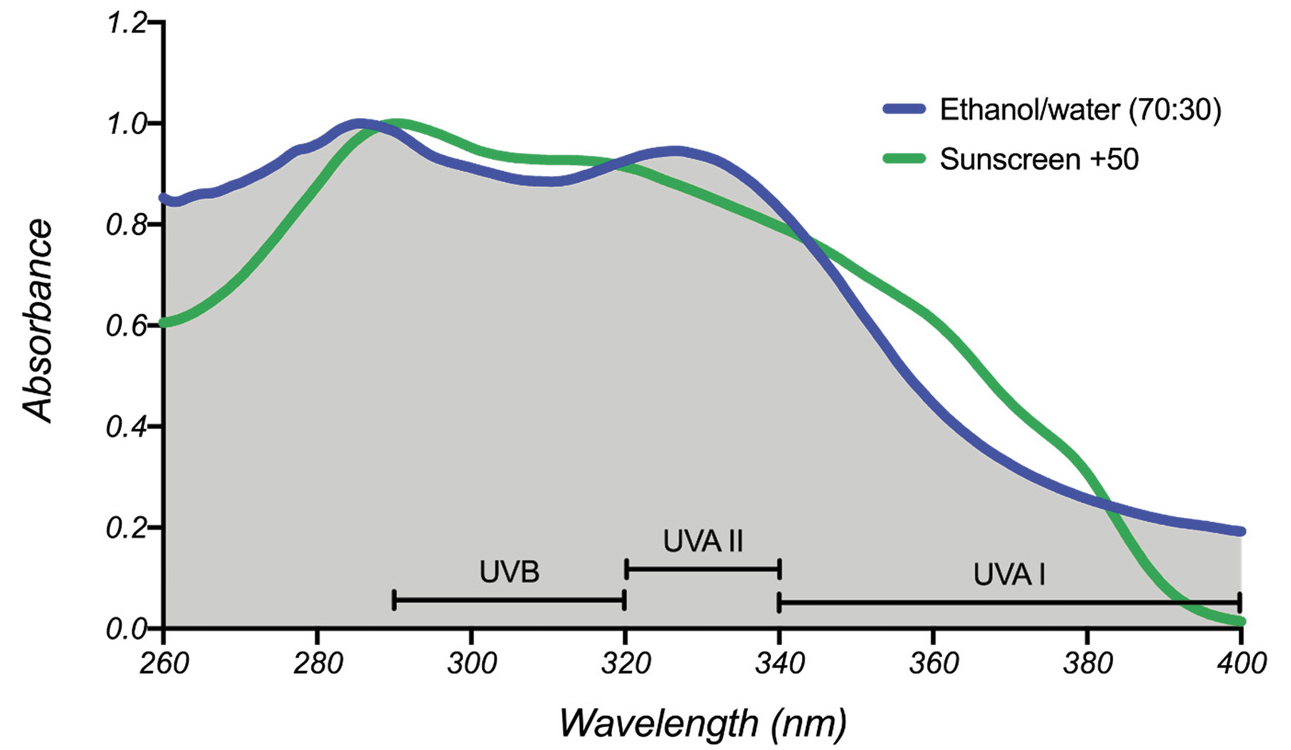
UV spectra of a commercial broadband sunscreen and an ethanol-water basil extract.
Assessment of the PBL sequence
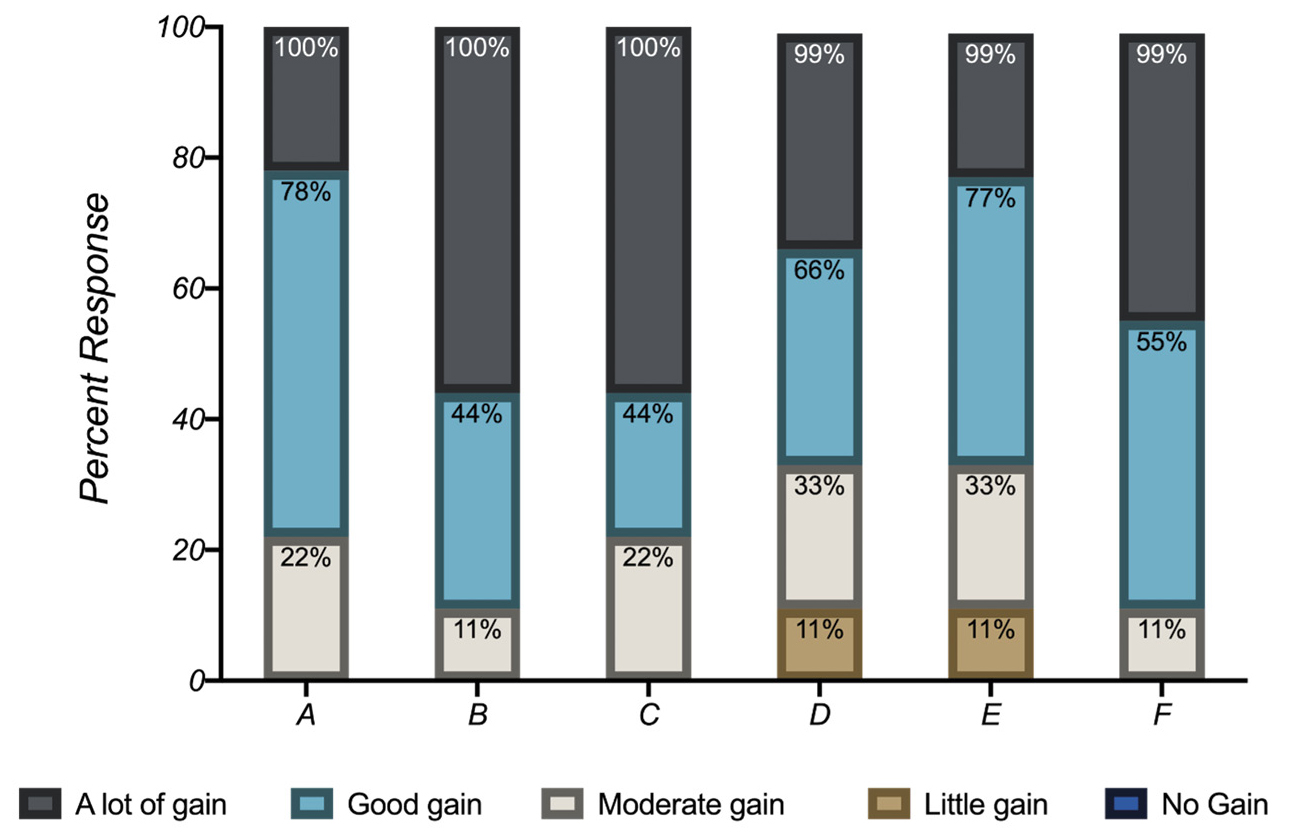
An opinion-blind and voluntary Student Assessment of their Learning Gains (SALG) instrument was administered over the spring and fall 2019 semesters. A total of 22 students submitted surveys. Due to the small sample size, our aim was not to develop a statistical instrument of the PBL experience. Surveys were designed to obtain feedback on students’ perception of their ownership of the material, their attitude toward STEM subjects, and their gains as a result of participating in this PBL sequence (Figure 7). A sample question with possible responses is shown in Figure 8. In addition, students were given the option to provide their opinions of the PBL experience. Here are some of the comments that students shared about their PBL experience:
Student Assessment of their Learning Gains (SALG) survey.
Sample survey question for students.
- As a result of participating in the PBL sequence, what gains did you experience in the following subjects?
- Your ability to summarize in scientific format literature papers.
- UV spectroscopy principles and their relation to concentrations of solutions.
- Your ability to identify patterns in data.
- Your enthusiasm for the subject.
- Your comfort level in working with complex ideas.
- Your willingness to seek help from others, instructors, TA, and peers, when working on academic problems.
- “I have learned how to use many different techniques, I have enhanced my mathematical skills, and my laboratory skills.”
- “I actually was very worried about the class in terms of the difficulty of concepts, but after taking the class I am confident about the materials moving forward.”
- “At first, I had a lot of assumptions about this course because I thought it would be really hard. After attending the lab session, I understood it wasn’t as hard as I originally thought.”
- “I have learned to accept help when needed, to help others when I can, and to share my knowledge to help build others’ understanding.”
Students reported a positive perception of their participation in the PBL sequence. We compared the feedback from students with our own observations. Students considered working in groups of two to be an advantage, and we observed that doing so encouraged discussion and collaboration among high-performing students and their peers. We provided multiple resources to accommodate the needs of all students, such as experimental handouts, literature papers, PowerPoint lectures, and direct hands-on instruction. The experimental handouts were generally praised by students. Students showed confidence in their literature search and writing skills coming into this project. Students prepared visually attractive presentations in PowerPoint and showed enthusiasm for exploring the literature on the environmental and health impacts of HCAs.
Although the enthusiasm was not lacking, students required help with the mathematical analysis of data and plotting of graphs in Excel, with the exception of a few high-performing students. Initially, we planned to have students calculate values of SPF, UVA/UVB, and critical wavelength λc independently. Most students were not able to complete this task on their own. However, we were pleased that students were successful in collecting accurate UV spectroscopy data, which fit nicely into a coherent scientific conclusion. To conclude their PBL sequence, students were asked to present the results in front of their peers. We wanted students to experience the emotions of talking directly to a professional audience. This activity replaced the final practicum for the laboratory class. We rewarded students for preparing engaging and visually appealing presentations rather than for the strict accuracy of their conclusions.
Discussion
A successful PBL sequence must incorporate creative features, which address our learning goals (Corwin et al., 2015).
- A PBL sequence must involve solving a real-life problem that students will perceive as authentic. Finding nontoxic alternative sources to potentially toxic commercial UV filters certainly piqued students’ interest. Students spent considerable time searching the literature on health benefits of HCAs using available search engines, including Google, and open-access publications.
- A PBL sequence must generate multiple hypotheses that lead to discussion and interaction. There were two associated hypotheses that students were expected to address: (i) whether a given ethanol-water mixture will produce an extract rich in HCAs from dry basil leaves, and (ii) whether the extract exhibits broadband UV screening properties. Furthermore, students could generate multiple hypotheses. For example, they could ask the following questions: Will the same results be observed if another plant is used? Will changes in the composition of the extracting solvents affect the results? Are time and temperature of extraction relevant? These are all issues that were raised during discussions with students and could become part of future CURE or PBL projects.
- A PBL sequence must incorporate knowledge and skills that satisfy curriculum requirements. Our PBL sequence was based on topics in our curriculum such as solid-liquid extraction, TLC analysis, and UV spectroscopy analysis. The sequence covered the prerequisite knowledge and added scientific context to experiments beyond curricular requirements.
- A PBL sequence must lead students to use problem-solving skills and creative thinking. Through the acquisition of data, students were asked to solve a real-life problem. They had to understand the relation between the concepts of polarity, miscibility of solvents, solubility of organic compounds, and molar absorptivity ε to generate meaningful conclusions using the data. They were exposed to advanced concepts such as the Beer-Lambert law, calibration curves, and assays, which are useful in higher-level biochemistry courses.
- A PBL sequence must be integrated and contain components of more than one discipline. We included elements of organic chemistry, analytical chemistry, and environmental and health sciences. Students were excited to learn about naturally occurring metabolites in the context of health and environmental impact. Students felt engaged during this activity, which was reflected in the time and effort they spent in preparing final presentations.
This PBL sequence provided us, the instructors, with a deeper understanding of the challenges that our students face. Mathematical manipulations, the use of Excel programming to plot visually informative and appealing graphs, and the connections between theoretical concepts and experimental data are areas we need to address better in the future.
Conclusions
A 4-week PBL sequence was embedded in the standard 1-semester curriculum of the Organic Chemistry I laboratory. The PBL sequence was designed to fit into the current curriculum, rather than to replace it. The short sequence format worked well for classes with a maximum enrollment of 18 students, which are typical of our organic chemistry laboratory curriculum. The PBL sequence can be applied to larger cohorts; however, coordination between instructors would be required so they could agree on a common theme and organize an efficient workflow.
The PBL sequence was designed to produce attainable results to serve as an adequate tool for teaching higher-order thinking skills. We chose a scientific inquiry that would stimulate students’ curiosity and produce useful data. Identifying nontoxic UV filters from natural sources is a current topic that addresses an important issue of environmental and health concern. Through this PBL sequence, we identified the composition of ethanol-water mixtures that produce basil extracts enriched in hydroxycinnamic acids, which could serve as broadband UV screening additives. As such, this PBL sequence has already produced useful conclusions, in addition to providing a platform for students to engage in collaborative laboratory research within curricular confines.
Most important, the PBL sequence served its purpose of improving the attitude of students toward STEM subjects. Students were excited to learn new skills and experimental techniques. In addition, students motivated their peers to obtain accurate results. The data collected by students fit nicely in two poster presentations, which were presented by representative students at the conference of the Metropolitan Association of College and University Biologists, held in October 2019, and at the Westchester Undergraduate Research Conference, held in April 2020.
One of the goals of this PBL sequence was to train students in laboratory research early in their studies to encourage them to participate in STEM research opportunities. We were very pleased that a few of our students, upon completion of the PBL sequence, asked to participate in further research work under the direction of the instructors.
Finally, we wanted to create a long-term, sustainable platform for collaborative work between students and instructors. The PBL sequence in the current format could lead seamlessly into other projects. In the future, we plan to catalog UV screening data for a series of aromatic plants with cosmetic and medicinal properties. Students can select a plant of their choice as a source of HCAs, study multiple extraction conditions, perform detailed HPLC analyses, and use a combination of assays to characterize the antioxidative capacity of plant extracts. We are planning follow-up PBL sequences in the Organic Chemistry II laboratory that will cover condensation reactions and esterification reactions, which are part of the traditional curriculum. Aldol and Knoevenagel condensations can be used to produce novel UV filters based on modified HCAs. In addition, data and products collected through PBL sequences can be analyzed further in summer research projects so that we can publish peer-reviewed research papers with student participation.
In conclusion, the short PBL sequence model can provide an effective, active, and inclusive learning platform for all students who wish to participate in meaningful laboratory research.
Ilirian Dhimitruka (dhimitru@uwp.edu) is an assistant professor of organic chemistry in the Department of Chemistry at the University of Wisconsin-Parkside in Kenosha, Wisconsin. Geetha Surendran (gsurendran@mercy.edu) is an associate professor of chemistry in the School of Health and Natural Sciences at Mercy College in Dobbs Ferry, New York.
Chemistry Labs Pedagogy Teaching Strategies Postsecondary


