Feature
Interdisciplinary Lessons on Energy and Entropy
Journal of College Science Teaching—May/June 2022 (Volume 51, Issue 5)
By Mika Munakata, Ashwin Vaidya, and Dirk Vanderklein
This article presents ideas and narratives of an experiment on the concept of energy developed for an honors seminar on energy and a mechanics course. We argue that energy is an idea best taught in an interdisciplinary manner. While most physics courses explore ideas such as mechanical energy and conservation of energy, it is imperative that a more practical view of energy as fuel be addressed since this has particular relevance to our students’ future. We believe energy cannot be discussed without talking about entropy and that a proper introduction to energy can only be made by simultaneously discussing the idea of entropy. In this article, we present one lab activity related to carbon emissions from bicycling, designed to help elucidate this idea through the use of conceptual metaphors and embodied learning. The learning goals of the activity were to encourage students to understand that energy and entropy are intricately related and to have students gain a deeper understanding of the concepts by considering and discussing ways in which they apply to different contexts. We discuss the rationale, implementation, and outcomes of the activity.
Despite the focus on the concept of energy in university physics courses, studies show that serious misunderstandings of this concept exist among students (Doménech et al., 2007; Duit, 1987). Among the problems identified are the difficulty students have with conceptualizing energy and students’ confusion of energy with force. Some students also see energy through a “materialist” view (Warren, 1982) by interpreting it as a fluid that resides in a body (Black & Solomon, 1983; Watts, 1983). Students’ understanding of energy and energy conservation also is often tied to mechanical ideas because students usually first encounter this concept in a course on Newtonian mechanics. This understanding may prevent students from developing a more generalized and sophisticated understanding of the concept.
In this article, we describe our interdisciplinary approach to helping students strengthen their understanding of the ideas of energy and entropy—concepts that are difficult to explain (Duit, 1987; Johnstone et al., 1977; Watts, 1983). Energy and entropy are typically defined from the point of view of work—that is, energy is the fuel needed to do work, while entropy is the wasteful by-product of doing work. We taught energy and entropy using an instructional module that had students explore entropy through metaphors situated in the context of carbon footprints; we found this to be a useful way to connect the topic to timely and socially relevant discussions about climate change. The work was motivated by our participation in several projects related to creativity in science and mathematics (Monahan et al., 2019; Munakata et al., 2021; Munakata & Vaidya, 2015; Vanderklein et al., 2016) and driven by the notion that learning occurs when connections are made and ideas are explored in context.
Historical perspectives on energy
The history of the concept of energy is mired in controversy. A look at the twisted paths taken and connections made that led to our current understanding of energy is revealing (Lehrman, 1973; Mach, 2014; Truesdell, 1976a, 1976b). While energy is typically presented in texts as the “capacity to do work,” Lehrman (1973) argued (quite accurately on historical grounds) that this definition is inadequate because energy cannot always be converted to “useful work.”
In fact, Lehrman (1973) noted that the theoretical formulas for energy emerged artificially as a means to unite the different branches of physics that developed between the 17th and 19th centuries. In the 19th and 20th centuries, energy found new meaning through developments in the fields of engineering, chemistry, and biology. The rapid growth and needs caused by the industrial revolution led to further investigations into energy for utilitarian purposes, which in turn gave rise to the laws of thermodynamics. These laws tell us that energy can change forms—thereby uniting the various new branches of physics and that not all forms of energy are available for work. Based on these advancements in the notion of energy, Lehrman (1973, p. 18) argued that the work-based definition of energy could be replaced by one in which energy is “described as the ability to produce heat.” He further stated that “a modern definition of energy, then, must be based on the first and second laws of thermodynamics. Anything less falsifies the picture.”
Given this history, we posit that a true understanding of energy is only possible if we consider the various facets of this abstract notion—including its history, mathematical formulation, technological significance, and meaning—from the perspectives of different disciplines.
Interdisciplinary perspectives on energy
In this article, we discuss interdisciplinary dialogues about energy. For example, in an honors seminar on energy taught by an interdisciplinary team of instructors, including one of the authors, students develop an understanding of the uses of energy, such as the energy required for an individual to flex their muscles or heat or cool a home, for a bacterium to move to its food source, or for the Sun to have the power necessary to serve as the ultimate energy source for all life on Earth. The course uses lectures, labs, and even field trips to help students understand what constitutes data and evidence related to energy. Students are also asked to evaluate the accuracy and reliability of information on contentious topics, including climate change. We have also found it useful to embed interdisciplinary ideas about energy in major disciplinary courses in physics and biology. In this article, we discuss one such interdisciplinary module that drew on our previous experiences and was implemented in two courses, an honors seminar course for nonscience students and a classical mechanics course for physics majors.
Teaching energy and entropy using metaphors
Studies have found that the concepts of energy and entropy are routinely misunderstood by college physics students (Christensen et al., 2009; Tatar & Oktay, 2007) and biology students (Halim et al., 2018). For example, while energy loss is mentioned as a way to define entropy, it is not dealt with in a concrete manner. It appears that many physics instructors fail to convey that energy and entropy are fundamental unifying concepts in science (Solbes et al., 2009). Entropy, arguably a central concept in physics, is often relegated to a course in thermodynamics. It is therefore important that we supplement our instruction of these ideas. Research points to the use of conceptual metaphors (CM) as a way to convey abstract ideas such as energy and entropy (Jeppsson et al., 2013). In the words of Lakoff and Johnson (1980, p. 195), CMs are ideas “which are understood and structured not merely on their own terms, but rather in terms of other concepts.” Three types of metaphors are commonly recognized: (i) orientational metaphors, (ii) ontological metaphors, and (iii) structural metaphors. A combination of orientational and ontological metaphors was routinely employed by the co-author Ashwin Vaidya in relating energy to the concept of money:
- Energy conservation: You start with $40 in your wallet. This is the amount of “energy” you have. This energy can be used to do useful work, such as purchase goods. Assume that no money is added or stolen from your wallet. If you were to use the $40 to only purchase food for $X and car fuel for $Y (energy conversion), you’d have $Z at the end. Then the statement $40 = $X + $Y + $Z is precisely the expression for energy conservation.
- Energy and entropy: Assume you have $100 (“energy”) in the bank and wish to withdraw a certain amount to make a purchase for $X (“work”) from an ATM that does not belong to your bank. In withdrawing the sum, you pay a small ATM fee of $e (“entropy”). As a result, you do not have access to the entire $100, but have an amount that equals $100 - $e. The fee that you end up paying is not retrievable directly and is a “tax” required to pay for the cost of maintaining the surrounding system.
These metaphors convey the general idea behind the physics concepts and can be used to lead to more precise technical definitions, formulas, and units that distinguish the various forms of energy and entropy.
The energy-carbon-footprint lab exercise described in the following section can be seen as an example of a structural metaphor, which refers to the relating “of one kind of experience or activity in terms of another kind of experience or activity” (Lakoff & Johnson, 1980, p. 486). The laboratory exercise has students operate a bike in an enclosed room. In this exercise, the metaphor for energy is the “fuel” used by the students’ vehicles or “food” consumed by the student, and entropy is explained in terms of carbon dioxide (CO2) released. Students measure and determine the net difference in CO2 emission during rest and bicycling. There are several advantages to using a metaphor in this physical way; active learning promotes a tacit and embodied understanding. The connection of abstract ideas to more relatable themes of practical significance also helps students build a long-lasting connection and promotes deeper understanding.
Carbon footprint lab: An example of a metaphor
Biologists and chemists are perhaps comfortable with introducing the ideas of entropy in their classroom. This is true especially in biology, where the concept is introduced indirectly. For example, in introductory courses, students study the carbon and energy cycles, which include discussions of pools and fluxes, and photosynthesis, and thus discussions about the conversion of electromagnetic energy from light into chemical energy in the form of carbon-based molecules. Because flux includes the loss of energy due to heat or respiration (Currie, 2011), students have been exposed to discussions about loss of energy. Our discussions about energy led to the development of a lab project, which is an extension of the development of the bicycle-generator designed by us for a previous classical mechanics course (see Appendix A online for the lab handout; also see Munakata & Vaidya, 2015).
The learning goals were to encourage students to
- understand that energy and entropy are intricately related, and
- gain a deeper understanding of energy and entropy by considering and discussing ways in which these concepts apply to different contexts.
The instructors chose a sufficiently small room of about 19.86 cubic meters and sealed it (see center image in Figure 1) to prevent the flow of air in and out of the room. A LI-COR 6400 infrared gas analyzer was used to record the baseline concentration of CO2 in the room (in ppm) with no one in the room. This step was followed by taking measurements for each of the three participating students when the student was in the room sitting on the bike but not pedaling (Case A) and when the student was pedaling at various speeds (Case B; see Appendix A). Recordings were taken every 2 minutes, for a total of 8 minutes. The recordings are shown in Figure 2a. For Case B, students were asked to pedal the bike at fixed rates (2 m/s and about 3.5 m/s, as shown in Figure 2b). Changes in CO2 emissions with increased rates of work were analyzed, in keeping with the metaphor of carbon emission as entropy (Suh, 2018).

Images from the lab on the carbon footprint of biking.
Note. The image on the left shows a student on a bicycle. The center image shows the sealed vent. The image on the right shows an image of the CO2 monitor.
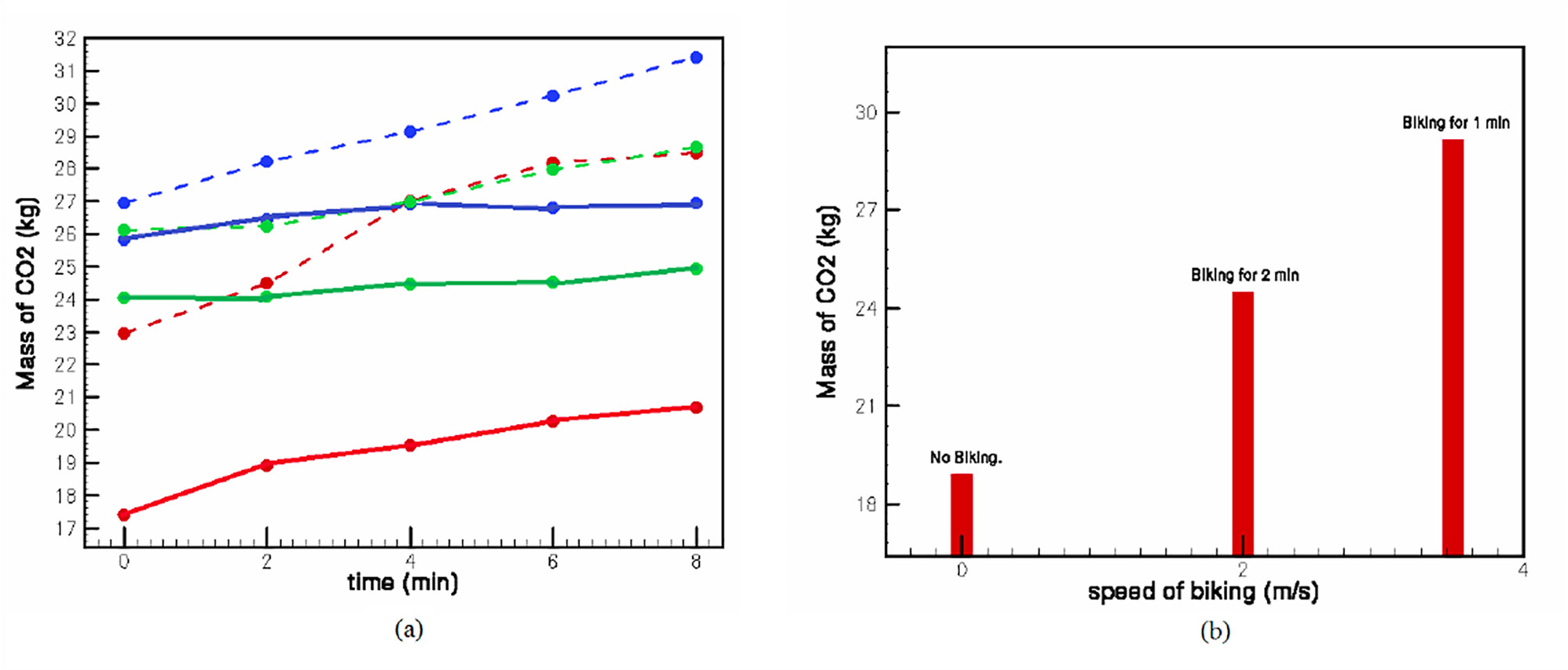
Results of the experiments on the carbon footprint of biking.
Note. The image on the left (a) shows the change in CO2 concentration in the room for different students in cases of no-pedaling (solid line) or pedaling (dashed line) at a steady pace. Each color (dashed and solid lines) represents a specific student participant in the experiment. The graph on the right (b) shows the average change in CO2 output, with increasing pedaling speed indicated in meters per second (m/s).
The experiment was conducted during a 2-hour lab session. Due to time constraints, three students (one male and two female) volunteered to participate in the exercise while sharing data with the rest of the class for analysis and discussion.
Figure 2a shows the mass of CO2 as a function of time for all three student participants (mass of CO2 = density of CO2 ˟ concentration of CO2 ˟ volume of the room; see also Appendix A). The solid curves indicate the CO2 measurements when the students were sitting on the bike without pedaling, while the dashed lines indicate the change in CO2 content as students pedaled at a steady pace (which was between 1.5 m/s and 2.0 m/s). The three colors used here (both solid and dashed lines) represent the three students who participated in the experiment. Figure 2b shows the concentration of CO2 as each student’s pedaling rate increased. The data shown are from one of the student recordings, but the other two showed a similar trend. The first two measurements were made after a 2-minute interval, while the last one was made after 1 minute. In between experiments, the door to the room and the vents were opened to release any lingering CO2 so as to not impact the results of the following run. We do realize the room may not have been perfectly sealed, which could have resulted in measurement errors. Nevertheless, the data appear to qualitatively validate the hypothesis of the study, indicating that in the process of bicycling, one tends to release more CO2 into the environment than they would when simply sitting still. Increased rate of work also causes an increase in the CO2 output.
Discussion
Students were given raw data on the concentration of CO2 in the room and asked to compute the relative mass of CO2 released into the room while someone was biking or sitting still. As part of this exercise, students were also asked to consider their annual driving habits and CO2 contributions. Students performed these calculations and compared them to the relative amount of CO2 they might release if they were to completely shift to biking. As their reports indicated, several students became aware of the fact that reliance on a machine comes with a price. They noted that (a) the fuel in their vehicles was the source of energy that (b) could be transformed through combustion to mechanical energy. These observations related to our first learning goal, which addressed the concepts of energy, entropy, and energy conservation. This exercise also addressed our second learning goal, as students realized the mere act of driving one’s car comes with a price—namely, carbon emission—which could be considered a form of the resulting entropy. Students came to understand that only a certain amount of the fuel used in their vehicles goes toward the positive (kinetic) energy needed for transporting their vehicles, giving them another, more practical way of understanding the energy conservation principle.
For students who owned a small or mid-size vehicle and drove approximately 12,000 miles annually, the increased CO2 production per year caused by their driving was more than 30 times higher than the CO2 cost of bicycling the same distance. Students deliberated on the disparity between the different modes of transportation and commented that while the cost of modern forms of transportation is considerably higher, a complete reliance on such a mode of transportation is not practical, given our energy needs and urban design. Therefore, students saw this exercise as one that increased their awareness about the practical side of energy (use), no matter how difficult it is to implement. Students seemed to agree that greater emphasis needed to be given to renewable energy sources or reduced consumption in order to reduce CO2 emissions. When assessing these responses, we concluded that our lab and ensuing discussions prompted students to gain insight into the ideas of energy and entropy, both from abstract and practical points of view. The average score for the students across the four labs was nearly 92% (lowest score = 88%, highest score = 99%), while the average score on the final exam (see sample questions in Appendix B) was about 88% (lowest score = 81%, highest score = 93%). The average scores reported are substantially higher than in traditional physics courses in which energy and entropy are discussed in a more standard manner. It may not be unreasonable, however, to infer from the average and distribution of scores that students came away from these experiences with a reasonable understanding of these concepts.
Time constraints prevented us from pursuing a more nuanced discussion about these topics, but stronger quantitative connections between different forms of energy could be made. Our second learning goal of interdisciplinary connections was also met through the lab exercise, which was introduced and conducted by both a physicist and a biologist. Before the labs, we discussed various ways to understand energy and the energy transfer processes that occur as food is converted to energy through metabolism, then used to do work, thereby converting chemical energy to the kinetic energy of the bicycle. In the future, we may have students analyze these conversions and make more explicit connections between biological and physical systems. Such calculations can improve students’ understanding of the different energy units such as calories, joules, British thermal units, and kilowatt hours. Understanding these units is crucial for students to be able to make connections between the topics discussed and their own lives.
We acknowledge that our learning goals are difficult to assess and are aware of the need to collect more data to determine outcomes related to specific learning objectives. In future implementations, we plan to analyze students’ work on specific assignments and collect data about the impact of our instructional strategies. For example, prompt-based student journaling could illuminate students’ development of ideas. Students could be asked to write about how ideas of energy and entropy play a role in their lives and how these concepts show up in other courses. Students could also be asked to develop new, creative metaphors for energy and entropy and write short narratives. In addition, open-ended homework assignments and tests incorporating similar questions could provide relevant data.
Conclusions
It is well recognized that interdisciplinary modes of learning allow for greater “cross-fertilization of knowledge” (Lawrence, 2010, p. 112). Such attempts seek to not only provide depth but also reveal what lies at the intersection of different disciplines, allowing for “innovative goals” and “enriched understanding” (American Association of Colleges and Universities, 2018). Our students were active in all phases of learning—from conjecturing through collecting data and revising methods to analyzing data and stating implications. Through this hands-on and multidisciplinary approach, students were able to take ownership of their own learning and make relevant connections that had meaning in their own lives.
It is particularly important that we use interdisciplinary approaches to bring issues surrounding energy and its implications to the forefront of the consciousness of the next generation. The global significance of energy was made clear by the United Nations’s declaration that the decade 2005–2014 be named the Decade of Education for Sustainable Development (Doménech et al., 2007). More recently, a report by the National Research Council, Climate Change Education in Formal Settings, K–14 (2012), discusses the significance of the climate crisis and argues that climate and energy education should be at the center of our efforts to deal with the issue. A relatively new and ambitious nationally funded program—the Climate Literacy and Energy Awareness Network (CLEAN), which involves collaborations between research (National Oceanic and Atmospheric Administration) and academic institutions (Carleton College, University of Colorado Boulder, Colorado School of Mines)—has been established to develop resources for teaching the science of climate and energy for Grades 6–16 (https://cleanet.org/index.html). By ensuring that learning takes place in an interdisciplinary and active environment while encouraging personal connections, we have attempted to highlight the important role each of us plays in sustainability efforts. We note, however, that while such small classroom attempts are important, we need to make greater strides in highlighting energy and entropy in the college or school curriculum across all disciplines to bring more diverse perspectives to the table.
Acknowledgments
This work was supported by funding from the American Physical Society’s APS Outreach grant (Vaidya and Munakata) and the National Science Foundation-IUSE program (Munakata and Vaidya, Award #1611876).
Ashwin Vaidya (vaidyaa@montclair.edu) and Mika Munakata (munakatam@montclair.edu) are professors in the Department of Mathematics and Dirk Vanderklein (vanderkleid@montclair.edu) is a professor in the Department of Biology, all at Montclair State University in Montclair, New Jersey.
Climate Change Interdisciplinary Physical Science Postsecondary


