research and teaching
Changes in Elementary Teachers’ Conceptions About Matter
Journal of College Science Teaching—November/December 2020 (Volume 50, Issue 2)
By Jerrid W. Kruse, Jesse Wilcox, and Lucas Menke
While research has investigated elementary teachers’ understanding of science content, such research is often limited to topics typically not addressed in elementary school curricula. Yet, research has illustrated that many elementary teachers struggle to accurately articulate and teach science concepts and professional development (PD) is needed. Therefore, this qualitative study sought to describe changes in in-service elementary teachers’ thinking about matter during a professional development targeting Next Generation Science Standards (NGSS) related to matter. Our findings indicate that participants entered the PD holding a wide range of conceptions, including those that were described as vague and inaccurate, as well as a variety of ways to articulate accurate conceptions. After the PD, participants’ vague and inaccurate conceptions typically improved to accurate conceptions, but the variety of ways to articulate accurate conceptions often reduced to only the ways discussed in the PD. Implications for teacher education are discussed.
As K–12 science classrooms around the United States continue to implement and refine their use of the Next Generation Science Standards (NGSS), elementary teachers have experienced obstacles when it comes to teaching science. In order for elementary teachers to fully enact the NGSS, teachers need to have a comprehensive understanding of content and pedagogical knowledge. However, studies have shown that elementary teachers are often lacking in understanding of science concepts and tend to be anxious or uncomfortable teaching science (Bulunuz & Jarrett, 2010; Cox & Carpenter, 1989; Saçkes, 2014; Tuttle et al., 2016). Because of this trend and the recent adoption by many states of the NGSS, we created a university-based professional development (PD) program to build elementary teachers’ science content knowledge. This paper focuses on a section of the PD program about matter concepts, in which we compare participants’ initial ideas about matter to their ideas after PD.
Rice (2005), using data collected over many years, noted elementary teachers clearly lack knowledge around fundamental science ideas. However, the questions used were only analyzed using “correct” and “incorrect” and do not reveal much about participants’ thinking. Specifically, numerous studies have found that elementary teachers cannot differentiate between chemical and physical changes and are unable to accurately articulate a particulate nature of matter (e.g., Gabel et al., 1987; Valanides, 2000). While these and other studies (e.g., Kokkotas et al., 1998) illuminate common difficulties elementary teachers have with understanding fundamental concepts about matter, they may not reflect the content necessary for enactment of the NGSS released in 2013. Therefore, exploration of teacher conceptual understandings directly connected to the NGSS may be useful for current efforts.
More recent investigations of elementary teacher knowledge often focus on the enactment of instruction and/or teachers’ pedagogical content knowledge. For example, Hanuscin et al. (2018) analyzed elementary teachers’ pedagogical content knowledge of properties of matter. They found that “teachers had difficulty interpreting the intent of the [NGSS] standards concerning teaching the small particle model (SPM)” and that “they had difficulty connecting the SPM with observable properties of matter and using it to explain phenomena” (Hanuscin et al., 2018, p. 19). While Hanuscin et al.’s study made some claims about elementary teachers content knowledge, the researchers’ data collection tools and methods focused on pedagogical content knowledge of the teachers. Smith et al. (2017) found similar results to those of Hanuscin et al. (2018) and noted that elementary teachers’ enactment of science instruction around matter “was rarely taught as envisioned by the NGSS… When teachers did teach about particles, their treatment tended to be purely descriptive rather than explanatory” (Smith et al., 2017, p. 15). Given these observed gaps in teacher knowledge, more exploration of how teachers conceptualize and learn NGSS-related concepts of matter may prove useful to university faculty responsible for helping elementary teachers gain the requisite knowledge for teaching about matter at the elementary level. While investigating pedagogical content knowledge is useful for methods instructors, investigating content knowledge may be more useful for university content instructors.
Purpose of study
Knowing that elementary teachers tend to be lacking in their understanding of concepts related to matter, we developed a PD program for elementary teachers that sought to help build pedagogical knowledge and content knowledge related to science. However, the current study focuses on elementary teachers’ content knowledge related to concepts about matter. More specifically, this qualitative study focuses on describing changes in participants’ thinking about NGSS-aligned ideas about matter.
Methods
This qualitative study used a case study framework so as to understand the case itself rather than generalize beyond it (Stake, 2005). However, case studies provide exemplars for the discipline (Flyvbjerg, 2006). Therefore, we have provided extensive details about participants and the PD so that readers might determine the extent to which the findings might transfer (as opposed to generalize) to their own contexts (Lincoln & Guba, 1985). The boundaries of the case are elementary teachers who participated in the 10-week physical science portion of a 30-week PD grant program. The case study is further bounded by focusing on participants’ thinking and conceptions of elementary concepts about matter within the NGSS.
Theoretical framework
This study used a theoretical framework of constructivism, which views knowledge as socially-constructed (Yin, 2003). Researchers using a constructivist paradigm often work to understand students’ perspectives rather than focus on an objective outcome. This paradigm provides participants opportunities to express their ideas (Crabtree & Miller, 1999), enabling the research to gain deeper insights into the participants’ understanding. For this study, participants had prior knowledge of matter that influenced how they conceptualized PD activities.
Sampling and participants
This case study used convenience and comprehensive sampling (Miles et al., 2014). That is, the PD studied was easily available as a resource to investigate in-service elementary teachers’ physical science conceptions and the sample included all PD participants. Miles et al. (2014) note that sampling includes aspects of setting, participants, events, and processes. The study’s setting was a 10-week physical science PD that was funded through the No Child Left Behind Title IIA program. The section on matter was part of a larger PD program, which consisted of a brief math course (six meetings), 10 weeks of physical science, 10 weeks of life science, and 10 weeks of Earth science. Each week of the science courses included approximately two hours of content instruction. Participants included 23 elementary teachers (21 female, 2 male) from a large, urban, Midwest school district. Three participants taught kindergarten, five taught first grade, three taught second grade, four taught third grade, two taught fourth grade, one taught fifth grade, one taught sixth grade, and four participants taught multiple grades (e.g., emerging bilingual support or special education). The process studied was the changes in participants’ thinking about matter concepts during the PD.
Professional development design
The PD program studied, as well as the pre- and postassessments, were aligned to the performance expectations of the NGSS and drew on principles of effective science teaching. For this study, we focus on the NGSS standards related to matter noted in Table 1. The PD coursework, described in detail in the next section, sought to model best practices with respect to the NGSS. Krajcik (2013) claims, “You cannot learn a core idea without using it with scientific or engineering practices. Therefore, using practices as a means to develop understanding of science ideas should be a regular part of students’ classroom experience” (p. 11). Bergman and Morphew (2015) note that content courses for teachers should model inquiry-based instruction (i.e., active learning) including hands-on activities and group investigations. The authors cite several benefits of such approaches to content delivery including: more realistic views of scientists, more accurate nature of science views, improved attitudes and confidence, and increased conceptual understanding. Therefore, all PD activities were designed to engage participants in collaborative investigation and problem solving using proper safety procedures (e.g., safety glasses, heat-resistant gloves). Furthermore, the PD program adopted a conceptual change approach (Pintrich et al., 1993; Posner et al., 1982) for instruction in which participants’ misconceptions about physical science were elicited and confronted through investigation with emphasis on helping participants make meaning of observations and data to develop accurate conceptions. Details of how each of the NGSS performance expectations related to matter were taught are discussed below.
| Questions on the matter assessment. | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Description of matter instruction during the physical science course
To address standard 2-PS1-3 in class, participants were asked to play with LEGO blocks and then discuss the connections between LEGO blocks and the concept that objects are made of small pieces that can be disassembled and made into a new object. As participants worked with the LEGO blocks, they were asked to create objects and then disassemble their objects to create new ones. After several iterations, participants discussed how different objects could be made out of the same number of LEGO blocks. They were then asked to extend their thinking by reflection on how the LEGO blocks example might apply to other things (e.g., buildings, automobiles, plants, animals). See Holub et al. (2020) for a more complete version of this activity with elementary-aged children.
In regard to standard 5-PS1-1, participants were engaged in an activity in which participants weighed a balloon with and without air. Participants then discussed what they thought contributed to the increase in mass as the balloon was filled with air, and what gave air mass. This discussion led participants to begin drawing “particle diagrams” in which they show the deflated balloon with very few particles of air by only drawing a few dots and an inflated balloon with many dots spread out inside the inflated balloon.
Standard 2-PS1-1 was addressed in the PD by asking participants to create a flowchart to organize different materials (e.g., nails, Styrofoam, rocks, pennies, water, oil, rubber bands). After sorting, we engaged participants in a discussion on the best ways to organize materials based on physical properties. Special attention was paid to helping the participants understand the problems with more extrinsic properties (such as size and shape) to categorize materials.
Standard 5-PS1-2 was taught by weighing a set amount of salt and water and then weighing the combination of salt and water. Before completing the mix, participants were asked to make predictions and explain their predictions to create active mental engagement. After mixing, participants then discussed what happened to the total number of particles to explain why the mass did not change upon mixing.
Finally, standard 5-PS1-4 was addressed through an activity in which we mixed baking soda and vinegar and had participants make observations. Participants quickly noticed that the mixture fizzed and bubbled, so we asked them to predict what might be happening to the mass and how they could investigate their prediction. After participants devised a way to mass the reactants pre- and postreaction, they came to see that the mass had gone down as they expected. Next, participants were asked what would happen if they captured the air being produced by the bubbles. Participants designed airtight systems (e.g., pop bottles, balloons) to see if the mass changed in a closed system. After a group discussion, participants, drawing on their knowledge from 5-PS1-1, discussed how the particles that left the system in the first iteration caused the mass to go down because those gas particles have mass. When participants captured the gas in the second iteration, the mass did not change. We then helped participants understand that this mixing must be creating a new substance that is able to escape as a gas. Specifically, we mixed baking soda and vinegar and asked what would happen if we added more baking soda. When the baking soda did not react with the liquid, we guided participants to realize the initial reaction chemically changed the substances.
Data collection
This study was ruled exempt by the Institutional Review Board as all data were collected as part of typical educational processes. All 23 PD participants were included in this study. The “matter” section of the PD program started with a preassessment, and ended with a postassessment containing the same questions. Each question of the assessment was aligned to NGSS performance expectations. Because this study sought to understand the depth and diversity of participants’ thinking, open-ended questions were used instead of closed-ended questions because open-ended questions have been shown to produce more diverse answers (Reja et al., 2003). Reja et al. (2003) note that to avoid nonresponse, open-ended questions should be explicit in their wording. Therefore, the questions often borrowed language from the NGSS standard language to more explicitly target the desired content. The questions and the corresponding NGSS performance expectations are given in Table 1.
Data analysis
Importantly, this research is not evaluative in nature. Instead, we sought to understand and describe changes in participants’ thinking. To do this, researchers analyzed each participant’s pre- and postassessment using process coding (Saldaña, 2013). Process coding uses gerunds to understand action in the data. The changes in participants’ thinking were conceptualized as actions and process coding served to identify ways in which participant thinking changed or “moved” from pre- to postassessment. Codes developed included: narrowing, simplifying, deepening, refining, focusing, modifying, and improving. By comparing these codes to each other and the data, the codes were reduced to narrowing, refining, and modifying. Once these codes were used to understand how participant thinking changed from pre- to postassessment, we engaged in operational model diagramming (Saldaña, 2013) to develop and refine a theoretical model encapsulating the changes we observed in participant thinking. Dominant changes in participants’ responses are reported in the results below using narrative description with pertinent codes bolded (Miles et al., 2014). Results of operational model diagramming are demonstrated in Figure 1 and also discussed in the results section below. To establish greater trustworthiness (Lincoln & Guba, 1985), all codes and diagramming were discussed between two researchers and quotes have been used throughout the results below to more clearly show how claims are supported by data.
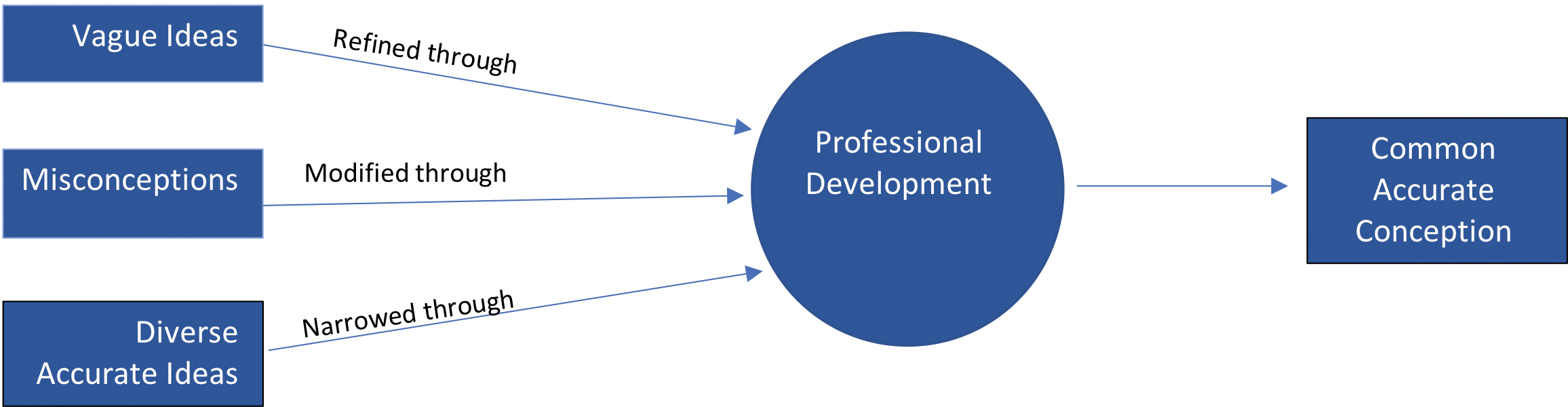
Impact of professional development on participants’ content knowledge of matter.
Results
Changes in cohort thinking with respect to each NGSS standard
This section provides a narrative description (Miles et al., 2014) to demonstrate common changes, or movement in thinking, within the elementary teacher cohort studied. Brief summaries along with supporting quotes illustrate common pre- and postconceptions. We have bolded the use of codes developed during the data analysis to highlight the nature of the most common changes noted within participant thinking within each concept.
Objects made of small pieces. As previously stated, the goal of this project was to analyze elementary teachers’ change in thinking about matter concepts after experiencing the PD program. To begin, we will look at the assessment question corresponding to standard 2-PS1-3, which relates to the concept that an object made of small pieces can be disassembled and built into a new object (see Table 1). While it is interesting that some participants considered how to demonstrate this concept to students, this study does not seek to make claims about participants’ pedagogical knowledge and these quotes still demonstrate participant thinking related to the concept that objects are made of small pieces.
On the pretest, many participants said that in order to demonstrate this concept they would use blocks, LEGO blocks, or similar objects to build and/or break apart structures. For example, one participant answered, “Break something apart [and] put it together in a different manner,” and another participant answered, “Use Legos (sic)–have students make a machine that will create a product. Then have them build a house with the same set of Legos (sic).” Using sand to demonstrate this concept was also a common response on the pretest. One participant responded, “Pour sand into a bottle. The sand fits the shape of the bottle.” Another participant answered, “(moon sand) Show that smaller parts pushed together makes something bigger.”
On the posttest, responses narrowed to focus almost exclusively on building and breaking structures with blocks. On this question, the basic idea of using blocks or similar items to build and tear apart structures did not change on the posttest. However, participants’ tended to become more specific in answering about the blocks. For example, one participant answered on the posttest, “Couple of thoughts when working with students—Use legos (sic) to build something larger—Draw or talk about how using smaller items (individual lego (sic) to build larger object; then ask how might change using same small pieces).” Another participant wrote, “I would use legos (sic). I would show the students legos (sic) put together to show an object and then a container of loose legos (sic) to show the smaller pieces.”
Particles too small to be seen. The second assessment question asked participants how they would demonstrate that air is made of particles too small to be seen, addressing standard 5-PS1-1 (see Table 1). On the pretest, participants’ responses were fairly scattered, but a few common demonstrations emerged from the data. For example, several participants focused on demonstrating that air takes up space. One participant wrote, “Put air in a balloon it takes up space but we cannot see it.” Other participants said that they would use a filter to demonstrate this concept, writing, “Put a white ‘filter’ on a fan and look at the discoloration after time.” Another common response on the pretest was to demonstrate using the relationship between fire and oxygen. One participant wrote and drew, “[Drawing of putting hand over candle flame to extinguish the flame] Air particles feed the flame on the left. There are plenty of particles for flame to consume. Cover the opening, and once those particles are consumed, no more fuel for the fire.”
After the PD program, the group’s thinking narrowed to demonstrating that air has mass, typically using a balloon as was demonstrated during the professional development. For example, a participant answered on the posttest, “I would weigh a balloon and then blow air into a balloon and ask students what they think is going to happen to the balloon’s weight. After weighing it we will discuss that the air contained particles that are now in the balloon.” Another participant on the posttest said she would demonstrate this concept “By weighing a balloon with and without air. When the balloon is full of air, the balloon weighs more.”
Describe and classify materials. The third assessment question addressed NGSS performance expectations 2-PS1-1 by asking participants how they might organize various rocks. The question was written to target the importance of intrinsic properties rather than extrinsic properties. Participants would typically list a combination of extrinsic and intrinsic properties when they answered on both the pre- and posttests. However, a few participants refined their responses to only intrinsic properties, writing properties such as hardness, density, and magnetism, and several participants reduced the amount of extrinsic properties (e.g., size and shape) from the pretest to the posttest.
Conservation of matter. The fourth assessment question addressed the conservation of matter (NGSS standard 2-PS1-2) by asking participants why after mixing a certain amount of substance A and substance B, the final product weighed less than the sum of the original two. On the pretest, participants provided a wide range of responses. The most common pretest response was to attribute this difference in mass to some type of phase change. One participant responded, “In the combinations some evaporated.” Most other responses attributed the difference in mass to absorption or dissolving, writing answers such as, “One substance maybe absorbed 2 grams of the other or maybe one substance evaporated,” and, “Substance A dissolved into B.”
After instruction, participants tended to modify their response to note that a chemical reaction between substance A and substance B produced a gas, and that the leaving gas caused the decrease in mass. For example, one participant answered on the posttest, “Substance A and substance B were mixed together and caused a chemical reaction. The particles were released into the air as the gas bubbles popped or fizzed. When the particles were released the mass dropped because the substances created a new mixture.” Another participant demonstrated a similar change in thinking, answering on the posttest, “Because a chemical reaction took place and part of it turned to gas and went into the air.”
Chemical reactions. The fifth and final assessment question builds off of the previous question by asking participants how they could know if a chemical reaction happened between substance A and substance B. On the pretest, many participants gave fairly vague answers about a change in the properties of the solution. Examples of these answers include, “Record the properties of A and B before mixing them. Record properties of the mixed substance. Compare and contrast results.”, “The properties would change and they would now be a different substance. They’d look different.” and, “The new substance would have properties that are different from the original substances.” Another common response on the pretest was to observe the ability to separate substance A from substance B after mixing them, inferring that if you can separate them after mixing, then a reaction has not occurred. As an example, one participant wrote, “If it was not possible to separate them back into substance A and B.”
After the PD program, the group narrowed their responses from vague, but plural, “properties” to focusing on mass change caused by formation of a gas. For example, one participant wrote on the posttest: When a chemical change occurs a new substance is created. Sometimes, for example when we combine the solid baking soda with the liquid vinegar a chemical reaction occurred and an invisible gas was created that wasn’t previously there.
Operational model diagram
To demonstrate the meaning we created from our data, we iteratively developed an operational model diagram to illustrate changes in participant thinking across NGSS content areas (Figure 1). When participants demonstrated misconceptions about a concept (e.g., conservation of matter), they typically modified their ideas toward a common accurate idea. Here we use the word “common” to mean that participants’ explanations and examples were similar to each other and often similar to what they had observed in the professional development. When participants started with vague ideas about a concept prior to the PD (e.g., describe and classify materials), they tended to refine their thinking to arrive at a common accurate conception at the end of the PD.
In the cases of misconceptions and vague ideas, the result of common accurate ideas is positive and somewhat expected given the PD was designed based on research in science education. Unfortunately, the outcome of a common accurate conception may not always be desirable. For example, when participants demonstrated diverse, accurate ideas at the preassessment (e.g., particles too small to be seen), the group’s thinking was often narrowed after the PD to one common accurate conception. While the overall movement of participants’ thinking toward common accurate conceptions is, at first glance, positive and somewhat expected, the narrowing of accurate ideas within the group is, to the extent we are aware, a novel observation.
Discussion
This study illustrates that PD targeting conceptual change through engagement in inquiry-based investigations can help elementary teachers develop more robust conceptions of matter aligned to the NGSS performance expectations. For example, many of our participants initially stated that one substance dissolving in another could cause the mass of the mixture to decrease. Yet, after the PD, participants noted that the production of a gas from a reaction from two substances would more likely cause the mass of a mixture to decrease. Clearly, elementary teachers often enter PD scenarios with misconceptions about matter (e.g., chemical reactions). These misconceptions are not surprising given literature observing that elementary teachers often have a lack of science content knowledge and tend to be uncomfortable teaching science (Bulunuz & Jarrett, 2010; Cox & Carpenter, 1989; Saçkes, 2014; Tuttle et al., 2016). While many college science instructors often resort to lecture-based approaches, this study provides additional evidence that more active learning approaches promote high levels of learning as has been noted in other studies (e.g., Armbruster et al., 2009; Laws et al., 1999; Paulson, 1999).
While teachers learning accepted scientific ideas is important, the “narrowing” code in this study illustrates an important caveat for science educators to consider when working with current and future teachers. On the pretest, participants’ correct answers tended to be fairly diverse. For example, on the preassessment many participants accurately demonstrated the concept that air is made up of pieces too small to be seen and they gave a wide variety of demonstrations they would use to demonstrate this idea. However, participants’ answers on the posttest tended to narrow to the examples given by the PD leaders. That is, at the postassessment nearly all participants stated that they would weigh a balloon with and without air to demonstrate that air is made up of pieces too small to be seen. This narrowing of the group’s answers may limit teachers’ ability to generate ways to demonstrate concepts to students. While teachers seem to have a strong understanding of one way to represent the concept, they may struggle to see how concepts can be explained or represented in many different ways. This limits their ability to represent or explain concepts to their students in multiple accurate ways.
This narrowing of diverse accurate answers toward a common accurate conception may indicate that our participants’ hold problematic epistemological beliefs that are mediating their learning (Figure 2). Epistemological beliefs are beliefs about the nature of knowing and learning (Easter, 2019; Hofer & Pintrich, 1997; Schommer, 1990). One possible explanation for the narrowing of some of our participants’ ideas may be that they believed PD leaders held the “right” answers. This notion relates to problematic epistemological beliefs such as learning and knowledge come from authorities (e.g., Schommer, 1990) and is simple rather than complex (e.g., Hofer & Pintrich, 1997). Participants may have erroneously believed they should not or could not deviate from the leaders’ approaches to demonstrating a concept or that there are single “right” answers to be learned.
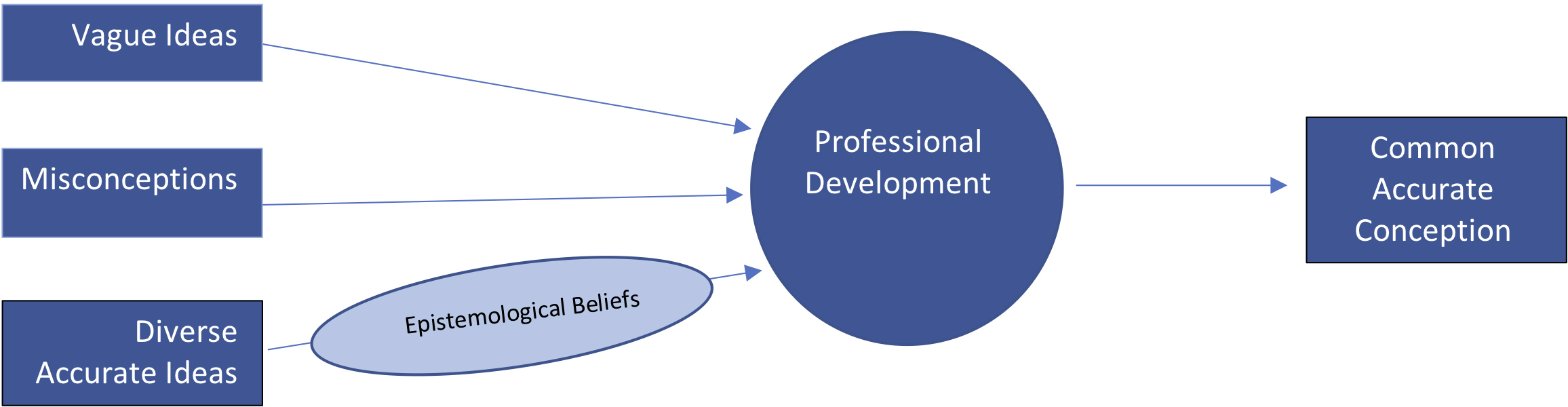
Theorized mediation of content knowledge by epistemological beliefs after a professional development on matter.
While narrowing participants’ ideas to a common conception was certainly not the intent of the program, the issue may be worth exploring further in future studies. Specifically, PD leaders might consider explicitly asking participants why other ways of explaining a concept, beyond those used in the PD, are of value. Furthermore, we wonder if more qualitative studies using open-ended questions might shed light on how educational treatments (e.g., PD, college science courses) affect the diversity of accurate answers provided. Given that teachers often must reframe or find different ways to represent content to help struggling learners, understanding several accurate ways to conceptualize science ideas could be beneficial.
Limitations
While this study has shed light on positive (e.g., modifying, refining) as well as negative (e.g., narrowing) ways participants’ thinking changed, all studies are limited. For example, we do not know to what extent the participants will apply their new understanding to their own teaching or to what extent our findings might apply to a different group of teachers, a different content area, or with a different PD approach. Furthermore, all researchers bring their own biases and dispositions to their work. For example, the bias created by using a constructivist theoretical framework led us to investigate the plurality of participants’ responses through qualitative methods and open-ended questions to better understand how participants make meaning. This particular choice, like all choices, has trade-offs. In this case, the transferability of our findings is difficult to determine. Therefore, we have worked to provide rich description and used participant quotes to make more transparent how meaning was made. While qualitative studies do not aim for generalizability, we hope data and discussion provided by this study spark reflection and ideas for those who teach science content to current and/or future elementary teachers.
NGSS Pedagogy Professional Learning old Teacher Preparation


