Middle School | Formative Assessment Probe
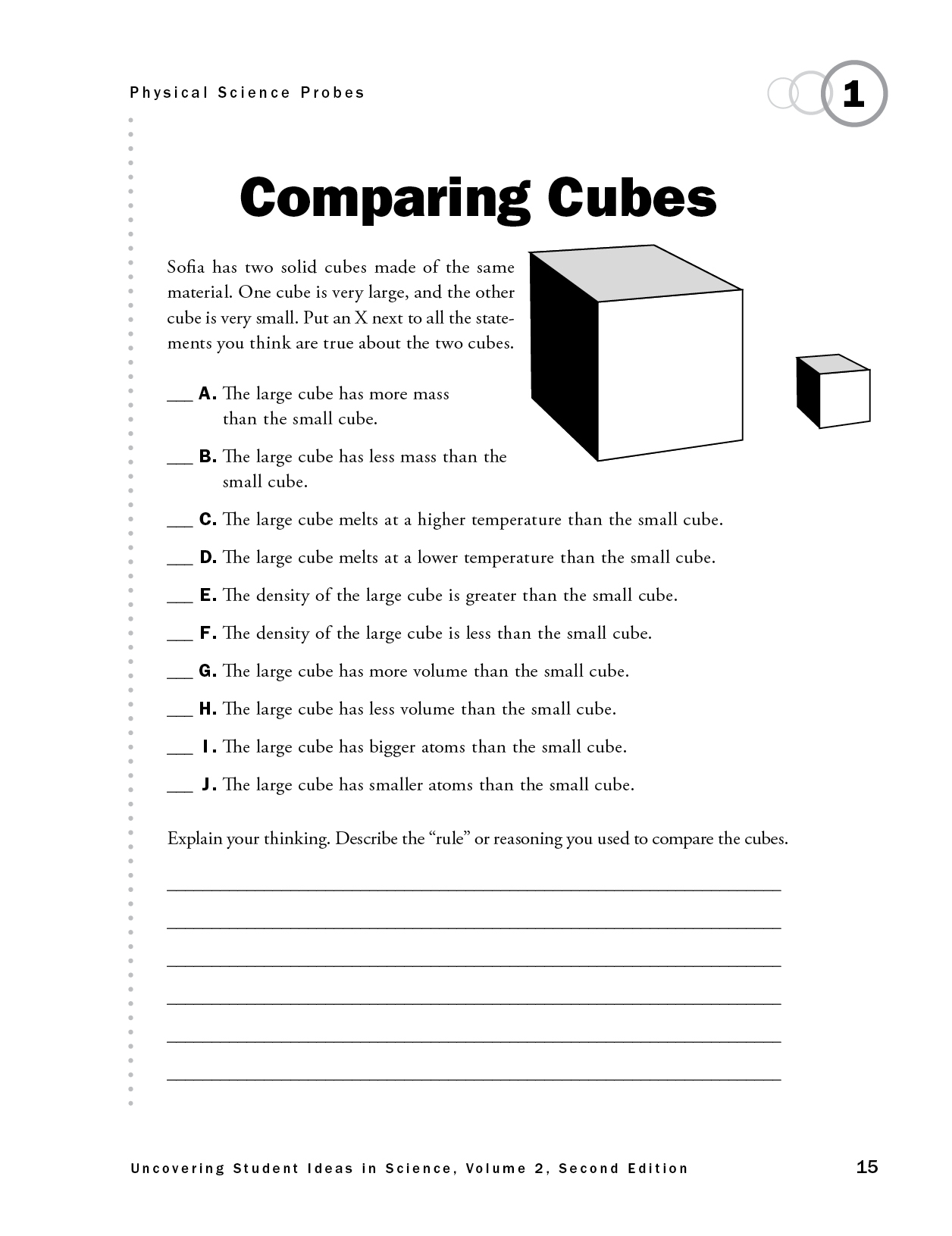
Comparing Cubes
By Page Keeley
Assessment Physical Science Middle School
Sensemaking Checklist




This is the new updated edition of the first book in the bestselling Uncovering Student Ideas in Science series. Like the first edition of volume 1, this book helps pinpoint what your students know (or think they know) so you can monitor their learning and adjust your teaching accordingly. Loaded with classroom-friendly features you can use immediately, the book includes 25 “probes”—brief, easily administered formative assessments designed to understand your students’ thinking about 60 core science concepts.
Purpose
The purpose of this assessment probe is to elicit students’ ideas about properties of matter. The probe is designed to find out which properties students think will change if the size of an object made from the same material changes.
Type of Probe
Justified list
Related Concepts
Atoms, properties of matter, extensive properties, intensive properties, mass, volume, density, melting point
Explanation
The best responses are A and G. Mass and volume are extensive properties that depend on the amount of matter. Mass is the amount of matter in an object, material, or substance. Volume is how much space the matter takes up. As the size of the cube increases, the mass and volume increase. Melting point and density are examples of intensive properties of matter. These properties stay the same for the cubes (under the same conditions) regardless of the size of the cube. For example, density is the ratio of the mass to the volume. If the mass of an object increases, its volume also increases proportionally. The size of the atoms remains the same, regardless of how large the object is. The large cube has more atoms than the small cube, but the size of the atoms stays the same.
Curricular and Instructional Considerations
Elementary Students
At the elementary level, students describe observable properties of objects, such as their size, weight, and ability to float or sink. Mass is a concept that is not introduced until later in elementary grades or in middle school. Weight is a stepping stone to mass in the elementary grades. The idea that the properties of weight and volume can change when the size of an object changes can be tested and observed by students. Melting point can be observed using familiar materials such as ice cubes or sticks of butter. Density and the size of atoms are concepts that should wait until middle school.
Middle School Students
In middle school, instructional experiences with the properties of matter progress from observational to conceptual, using a particle model of matter. Students learn that some properties, such as density and melting point, are useful in identifying and comparing different substances because they do not change with the amount of matter. Density is a particularly difficult concept at this level. An understanding of density progresses from the qualitative float and sink observations in the elementary grades to the quantitative proportional relationship between mass and volume at the middle school level. The particle model of atoms is still abstract for many students at this level. The probe is useful in determining whether students have preconceived ideas about atoms and whether students relate a macroscopic change in the size of an object, material, or substance to a microscopic change.
High School Students
Instruction at the high school level builds on the concept of characteristic properties of substances that was developed in middle school and integrates the details of atomic structure with how atomic architecture plays a role in determining the properties of materials. The terms intensive and extensive properties of matter are introduced. This probe is useful in determining if students are able to explain the distinction between intensive and extensive properties at a substance or particle level. The probe may reveal that high school students revert to their strongly held preconceptions even after they have been taught the concept of characteristic properties in middle school.
Administering the Probe
This probe is best used with grades 6–12. This probe intentionally does not mention the material that makes up the cubes because the type of material may influence students’ thinking. Be sure students understand that the cubes are solid and made up of the same type of matter. It may help to have visual props for this probe, such as two different sizes of blocks made from the same material or ice cubes. Make sure students do not focus on the particular type of material. They need to understand that the probe applies to any type of solid material, as long as both cubes are made of the same material (have the same composition) and are under the same conditions of temperature and pressure. Upper elementary teachers may find this probe useful if they substitute the word weight for mass and remove choices E, F, I, and J.
Related Research
- Many students age 15 and older still use sensory reasoning about matter, despite being well advanced in thinking logically in other areas, such as mathematics (Kind 2004).
- Ideas that interfere with students’ conception of density include the belief that whenyou change the shape of something, you change its mass (Stepans 2003).
- An intuitive rule of “more A, more B,” may cause some students to reason that if you have more material, properties such as melting point or density increase (Stavy and Tirosh 2000).
- Although some students ages 14–22 relate density to compactness of particles, incomplete explanations may result from their conceptions of mass and volume, which require understanding of the arrangement, concentration, and mass of particles. Many students have misconceptions about volume that present difficulties for understanding density (Driver et al. 1994).
- Students of all ages show a wide range of ideas about particles. Some students will attribute macroscopic properties to particles (AAAS 2009). For example, they may believe the size of the atoms that make up the cube increases as the size of the cube increases.
- A study of 60 Australian 11-year-old students found that more than 80% had misconceptions about volume that led to difficulty in understanding density (Rowell, Dawson, and Lyndon 1990).
- A study by Smith, Carey, and Wiser (1984) found that students’ earliest ideas about density may be described by the phrase heavy for its size. However, students fail to bring together the two ideas of size and “felt weight” so that density and weight are not differentiated but rather are included in a general notion of “heaviness.”
Related NSTA Resources
Grooms, J., P. Enderle, T. Hutner, A. Murphy, and V. Sampson. 2016. Argument-driven inquiry in physical science: Lab investigations for grades 6–8. Arlington, VA: NSTA Press.
Mayer, K., and J. Krajcik. 2017. Core idea PS1: Matter and its interactions. In Disciplinary core ideas: Reshaping teaching and learning, ed. R.G. Duncan, J. Krajcik, and A. E. Rivet, 13–32. Arlington, VA: NSTA Press.
NGSS Archived Webinar: NGSS Core Ideas— Matter and Its Interactions. Available at http:// learningcenter.nsta.org/products/symposia_seminars/ NGSS/webseminar27.aspx.
Peterson-Chin, L., and D. Sterling. 2004. Looking at density from different perspectives. Science Scope 27 (7): 16–20.
Shaw, M. 1998. Diving into density. Science Scope 22 (3): 24–26.
Talanquer, V. 2002. Minimizing misconceptions: Tools for identifying patterns of reasoning. The Science Teacher 69 (8): 46–49.
Suggestions for Instruction and Assessment
- This probe can be followed up with the science practice of planning and carrying out investigations. Have students observe, measure, and discuss what is the same and what is different about the mass (or weight), volume, melting point, and density of two different-sized cubes of a familiar substance (such as ice) that has a melting point that can be measured safely by students.
- The probe “Mass, Volume, and Density” in Uncovering Student Ideas in Physical Science, Volume 3 can be used to further uncover student thinking about these concepts (Keeley and Cooper 2019).
- Have students use the crosscutting concept of cause and effect to explain what happens to different properties when the size of an object changes.
- Probe further to determine if students use the same reasoning to explain what happens to different properties when the amount of a liquid or gas changes.
- Provide multiple and varied opportunities for students to observe and measure characteristic properties such as boiling point, melting point, density, and solubility using different amounts of the same substance.
- Have middle or elementary students test the idea that different volumes of the same substance usually have different masses (or weights, for elementary students). Then have them test the corollary that different masses (or weights) of the same substance usually have different volumes. Help middle school students relate each to a conceptual understanding of density, constructing their own understanding of the D = M/V proportional relationship (density equals mass divided by volume) before being given the mathematical equation.
- Have students use the crosscutting concepts of patterns and scale, proportion, and quantity to explain what happens to properties when the amount of matter changes. For example, identify patterns when comparing the density of the same substance when the mass changes and describe the proportional relationship.
- Be aware that teaching a specific characteristic property such as density by itself may not help students develop a unified idea of characteristic properties that includes density, boiling point, melting point, and solubility. Be explicit in developing and pointing out the idea that all these properties have something in common—they do not depend on the amount of the sample. Once students grasp this concept, introduce the terminology, intensive and extensive properties.
- Have students conduct an investigation to determine the melting point of a small, medium, and large amount of the same substance, such as ice, butter, or wax (using appropriate safety precautions).
- To help middle school students distinguish between characteristic and non-characteristic properties, hold a mystery object in your hand (closed). Ask students if they can tell you what the object is if you give them the weight or mass, color, shape, texture, length, volume, or other non-characteristic properties. Elicit ideas about what kinds of properties might be helpful to know in order to identify the mystery object. After developing the idea of characteristic properties through a variety of instructional experiences, revisit the mystery object in your closed hand. Ask the same questions about which properties would help them identify what the mystery object is. Use the information formatively to assess whether students have grasped an understanding of characteristic properties.
- Have students practice using “if, then” reasoning with physical properties to examine cause-and-effect relationships. For example, prompt them to respond to statements such as, “If the volume of a substance increases, then its boiling point ____ because ____.” This can be practiced with elementary students using basic extensive properties of objects. For example, “If the shape of a clay ball changes, its weight will ____ because ____.”


