Middle School | Formative Assessment Probe
Salt Crystals
By Page Keeley
Assessment Physical Science Middle School
Sensemaking Checklist




This is the new updated edition of the first book in the bestselling Uncovering Student Ideas in Science series. Like the first edition of volume 1, this book helps pinpoint what your students know (or think they know) so you can monitor their learning and adjust your teaching accordingly. Loaded with classroom-friendly features you can use immediately, the book includes 25 “probes”—brief, easily administered formative assessments designed to understand your students’ thinking about 60 core science concepts.
Purpose
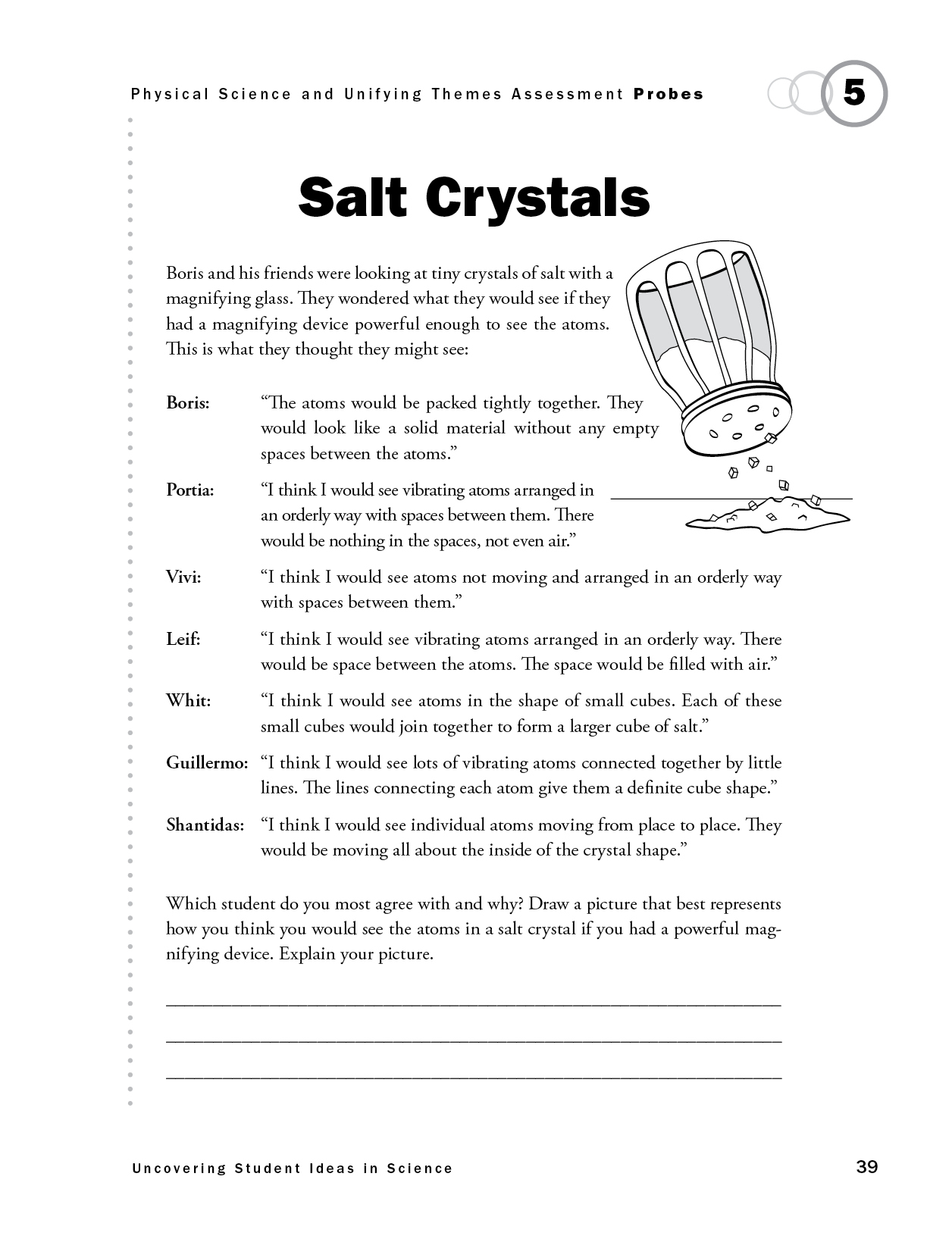
The purpose of this assessment probe is to elicit students’ ideas about crystalline solids. The probe is specifically designed to determine how students think atoms are arranged and move in a crystalline lattice.
Type of Probe
Friendly Talk
Related Concepts
atoms, crystal, crystalline lattice, ionic bond
Explanation
The best answer is Portia’s: “I think I would see vibrating atoms arranged in an orderly way with spaces between them. There would be nothing in the spaces, not even air.” Salt is an example of a crystalline ionic lattice. A salt crystal is made up of an orderly repeating array of sodium and chloride ions. This repeating array is caused by the electrostatic attraction between negatively and positively charged atoms called ions and forms the salt crystal’s distinct cuboidal shape. The tiny crystals are made up of the atoms (in the form of ions). They are in the form of a solid in which the atoms are closely locked in position and can only vibrate. They are not free to move around as in a gas. There is empty space between the atoms that make up the salt crystal. There is no air in these spaces because the material is salt (sodium chloride), not a mixture of salt and air. The crystalline matter is sodium and chlorine atoms only. Sometimes models, such as ball and stick models, depict sodium chloride (table salt) as a repeating cuboidal three-dimensional array of atoms connected by lines representing the ionic bonds. These lines are not actual physical structures but rather represent the attraction among the ions.
Curricular and Instructional Considerations
Elementary Students
In the elementary grades, students observe macroscopic properties of matter and details they can see using magnifiers. Their observations focus on the features of objects and materials. Using magnifiers, they can see that salt has a cuboidal shape. However, explaining that microscopic structure in terms of atoms exceeds expectations for this grade level.
Middle School Students
In the middle grades, students begin to use atomic and molecular ideas to explain phenomena and structural arrangements. They distinguish between molecular substances and crystalline lattices, although the details of ionic and covalent bonding can wait until high school. They should know that solids are rigid structures made up of atoms and that the atoms, with some empty space between them, can only vibrate in place, not move about.
High School Students
Students at the high school level should be able to use ideas about atomic/molecular motion to explain phenomena and structural arrangement from a microscopic view. They should be able to explain the difference between ionically bonded compounds and other types of chemical bonds. They frequently use ball and stick models to explain structure and behavior. However, even though they may understand what an ionic bond is, they may still hold on to misconceptions about the space between atoms.
Administering the Probe
This probe is most appropriate at the middle and high school levels. Consider having students examine grains of salt macroscopically before answering the probe.
Related Research
- Students of all ages show a wide range of beliefs about the nature and behavior of particles. For example, they attribute macroscopic properties to particles; do not accept the idea that there is empty space between particles, and have difficulty accepting the intrinsic motion of solids, liquids, and gases (AAAS 1993).
- Children frequently consider atoms of a solid to have all or most of the macroproperties they associate with the solid (Driver et al. 1994).
- Twenty-eight Australian 17-year-olds were interviewed in an Australian study conducted by Butts and Smith (1987) that focused on the formation of sodium chloride and use of the ball and stick model. The students referred to molecules of sodium chloride and stated that there were ionic bonds between the molecules.
Related NSTA Resources
American Association for the Advancement of Science (AAAS). 2001. Atlas of science literacy. Vol. 1. (See “Atoms and Molecules” map, pp. 54– 55.) Washington, DC: AAAS.
Logerwell, M., and D. Sterling. 2007. Fun with ionic compounds. The Science Teacher (Dec.): 27–33.
Robertson, W. 2007. Chemistry basics: Stop faking it! Finally understanding science so you can teach it. Arlington, VA: NSTA Press.
Suggestions for Instruction and Assessment
- Use magnifiers to see the cuboidal shape of salt crystals. Challenge students to think what these cubes would look like at the microscopic level of the atom. Help them distinguish between the properties of the material (salt crystal) and the properties of the atoms. Just because a material has a certain shape does not mean the atoms have the same shape.
- A variety of materials, including ball and stick models, can be used to illustrate the ionic arrangement of the sodium and chloride atoms in table salt. However, make sure students do not think the sticks are actual physical structures between atoms.
- Clarify the difference between an ion and an atom.
- The PRISMS (Phenomena and Representations for Instruction of Science in Middle Schools) website at http://prisms.mmsa.org has a collection of reviewed web representations to help students visualize the atoms in a crystalline array. This website is part of the National Science Digital Library and can also be accessed through http://nsdl.org.


