Science 101
Q: Do Scientists Really Use the “Scientific Method?”
A: Before I tell you (spoiler alert) that the answer is “no,” I want to first point out the problem of how science has been traditionally taught—in a way that does not express the fascination of science and does not sufficiently convey the excitement and creativity of the scientific enterprise to create a strong interest in science and encourage students to continue learning science and consider a career in one of the sciences.
We do a disservice to—and misrepresent—science if we teach it as merely a collection of facts, or that its undertaking requires following a rigid procedure. Many teachers describe a “scientific method” to students, which typically involves somewhere from four to eight steps. The basic steps are usually presented as something like: question, hypothesis, experiment, and conclusion. This is how many scientific papers are structured, in which the author presents information for readers in that order. But the actual research probably took place very differently. See the article in Online Resources on “Problems with ‘The scientific method’” for a nice description of the traditional “scientific method” compared to what scientists actually do. It also includes examples of better types of scientific investigations you can do with your students.
We can look at different ways that a scientific investigation can start, different ways it might be carried out, and the variety of—often not discussed—activities that come before or after the traditional steps. If you look at some famous scientists’ notebooks, you don’t see any standard sequence of steps. You see numerous ideas, plans, blind alleys, calculations of one kind or another, notes from other, related studies, etc. Is it starting to sound like real science is a bit messy? It should, because it is. And that messiness is a natural consequence of the creativity and fun that is part of real science. (I’m sure I don’t have to tell you that fun and messiness often go hand-in-hand.)
The Start of an Investigation
Many scientific studies start with an observation, which leads to a question. It could be an observation of something in nature, such as seeing that the sky is blue (leading to the question, “Why is the sky blue?). Or it might have been an observation in a previous experiment. For example, a student is observing how the temperature of water rises as water boils (and never goes above a certain temperature). But, in the process, she observes that long before the water boils, little bubbles appear in the water at the bottom of the pot. Why did those bubbles form before the water reached the boiling point? Or, someone might just wonder about something they heard. “Pat says that a heavier rock falls faster than a lighter one. I wonder if that’s true.” So then Pat tries dropping two different-size rocks and discovers that they fall at the same rate. This leads to the question, “Why didn’t the heavier rock fall faster?”
Notice that the essential questions started with “Why.” See the sidebar on how scientists usually try to answer “Why...” questions, rather than “What...” or “Which...” questions. Scientists seek explanations for what they observe. And when they suggest a possible explanation (which can be tested), they call that a hypothesis. Notice how this is different from the usual way that the term is defined by teachers. They often describe it as a guess (or an educated guess) at the outcome of an experiment. But that’s not a proposed explanation and not what scientists typically do. For more information about just what a hypothesis is, see the hypothesis article in Online Resources.
Also note that many science experiments do not need a hypothesis, so we shouldn’t require one. For example, suppose a student wants to know how tall the big tree near her home is. Is there a way to measure that? (Yes.) But making a guess at the answer won’t help in any way to get the answer (which is why scientists don’t do that). And this is not a case where a scientific hypothesis, as a possible explanation, is needed. The student just needs to investigate procedures that people use for this type of measurement and then can try doing it herself. Lastly, note that the NGSS science and engineering practices do not include anything about a hypothesis.
A Better Description of Science
Does all this sound like heresy? Is it surprising that I’m downplaying the hypothesis? Does it seem strange for me to say not to use that five-step “scientific method?” A resource that does an amazingly good job at describing how science works is the Berkeley “Understanding Science” website. A link to it is in the Online Resources. It also provides science teaching materials for every grade level.
If that standard “scientific method” is not what scientists actually do, why is it taught so widely? Probably because it’s easy to teach and easy to test. But that doesn’t make it right. What to do instead? What is the real scientific method? In one sentence, here’s what scientists do: Scientists ask questions about anything in the world (or universe) and then look for pathways to answers. After finding out what’s already known about the topic, they get more information in a variety of ways—by making observations, by undertaking scientific experiments/investigations, and by making calculations. So, there’s not a single “method;” there are many scientific methods. You can see more about these various methods in the Process of Science publication listed in Online Resources.
An Investigation
Let’s investigate something and learn something new! Have you noticed that people often wear darker-colored clothing in the winter and lighter colors in the summer? Why is that? Might darker-colored clothing somehow keep you warmer in winter? Might lighter-colored clothing keep you cooler in the summer?
Ask students for ideas on how one might answer these questions. Some may suggest going outside on a sunny day wearing some light-colored clothing, and then again with some dark-colored clothing and report which clothing made them feel warmer. But that’s a very subjective judgement, lacks controls, and is susceptible to bias. Another suggestion might be to use (identical) thermometers covered in different colors of cloth out in the Sun. Will a thermometer under one color cloth record a different temperature than thermometers under a different color?
Scientific instruments like thermometers allow our young scientists to make unbiased measurements. In this way, scientific instruments are the great equalizer; a person from any culture, from any demographic group, will record the same readings—and can check each other’s readings. (This is one reason why international scientific collaborations work so well. Indeed, the largest science projects, from the International Space Station to the Large Hadron Collider, are all international endeavors.)
Here’s another approach without using thermometers. You’ll need some scraps of cloth—two or three black or very dark-colored pieces, and two or three white or very light-colored pieces. Go outside on a sunny day with some small plates or bowls—as many as you have pieces of cloth—and some ice cubes. Put one ice cube in each plate or bowl and cover it with a piece of cloth (see Figure 1). Record your observations, noting which ice cubes, under which color cloth, melted faster and which melted more slowly.
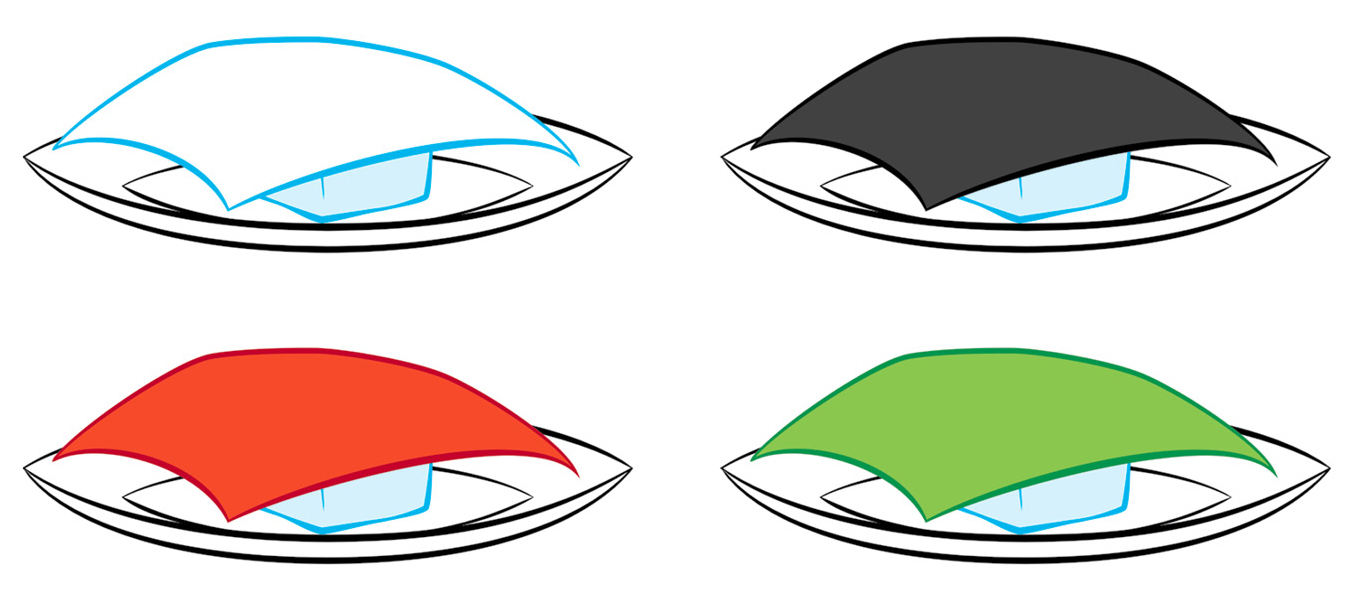
Ice cubes under different colors of cloth.
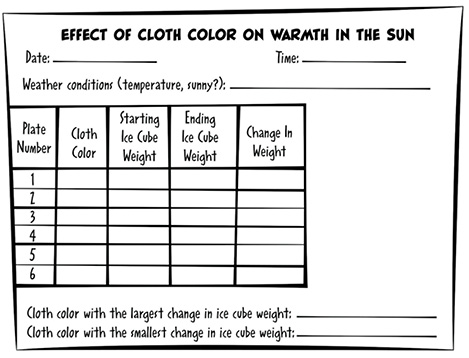
If you want to be more precise and include additional measuring skills, weigh each ice cube at the beginning. Make a table of observations with a line for each plate/ice cube showing its initial weight (including units!) and the color of cloth placed over it (See Table 1). You don’t have to wait for all the ice cubes to melt. After 10 or 15 minutes, weigh each ice cube again and record the weights in the table. Do the subtraction to find out how much ice melted (last column in the table).
Have students look at the completed table and discuss which color cloth caused which ice cubes to melt the most. What can they conclude about wearing dark or light colors in summer or winter?
The Real Science: Explain Why
So now we’ve answered the question and we’re done, right? Hold on, because we’re just getting to the most important part of the science. That experiment is just part of the investigation. Before we finish the investigation, notice a few things about what we’ve done so far—or haven’t done. One thing that we didn’t do is have students start by guessing at the outcome of the experiment. Why not? Because that wouldn’t help us to answer the question. Secondly, while we’ve observed that dark colors make the ice melt faster, we haven’t discussed why that happened. Answering that “Why?” question is where the real science occurs. This is the point where we might want to make a hypothesis—a tentative explanation for what we observed.
To guide students toward an explanation, discuss with students where our warmth—or, at higher grades, heat energy—comes from. Many will probably say that it comes from the Sun (especially since we did our experiment out in the Sun). That’s correct, but let’s look at the process in a little more detail. What comes from the Sun is light—both visible and invisible forms of light. Some of that light gets reflected or scattered by the cloth. That reflected light is what enables us to see the cloth! So, light from the Sun reflects off the cloth, but only certain colors of light reflect off the cloth and enter our eyes. If the cloth is red, then it reflects mostly red light, and that red light enters our eyes, which enables us to detect the red color (see Figure 2).
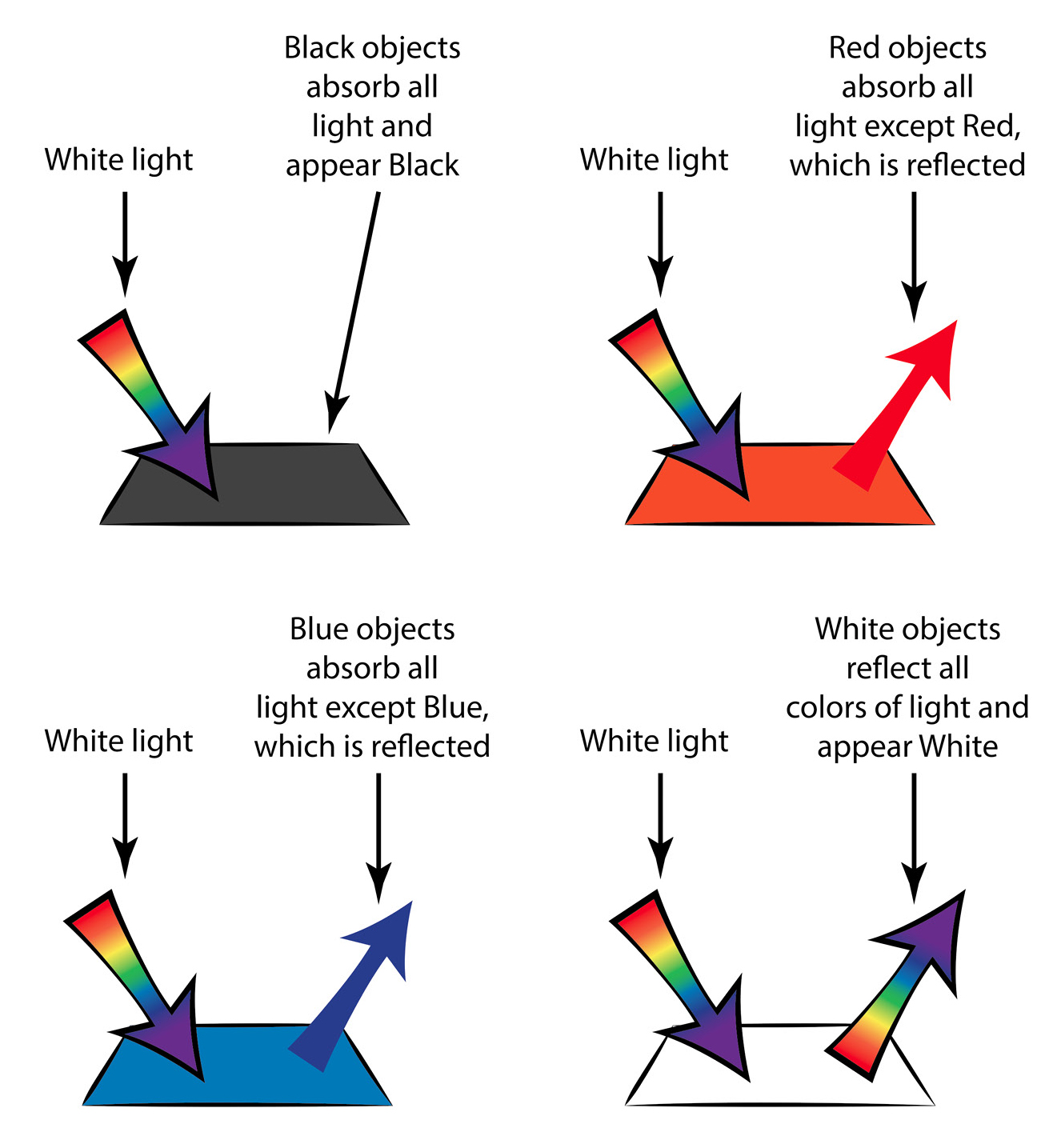
The white light from the Sun includes all colors, but only certain colors get reflected. The reflected colors determine the color that the object appears to our eyes. (While basically correct, this figure is a bit of a simplification, as more than one color can—and usually does—get reflected from a colored object. Also, no objects are absolutely black, so a tiny amount of light does get reflected from “black” objects. However, these nuances are not important at the lower grades.)
White cloth reflects all colors, and when all colors combine in our eyes, we see white. Black cloth doesn’t reflect any colors (or very little), so it appears dark. But what happened to the light that wasn’t reflected, like blue light in the case of the red cloth, or pretty much all the colors in the case of the black cloth? That light gets absorbed, meaning that the light waves stop, and the light energy gets converted to heat energy. So, the more light that gets absorbed (and the less reflected, i.e., the darker the cloth), the warmer the cloth will get.
Think back about what we did in this investigation. We asked a question and then did an experiment to answer the question, but that didn’t really teach us very much. The real learning—the real science—came in finding an explanation for what we observed. That is the essence of science—finding explanations for why the world works the way it does.
Scientists Ask “Why,” not “What” or “Which”
At some science fairs that I’ve judged, I’ve seen projects like “What (or which) brand of paper towels is strongest?” However, that’s not usually the kind of question a scientist would be investigating. A scientist (perhaps employed by Bounty to create the strongest paper towels) would observe that brand XYZ paper towels are strongest and then ask Why are those the strongest? Scientists seek explanations, and it’s the “Why” questions that demand answers in the form of explanations.
In this case, if the student is interested in the strength of paper towels, after finding out that XYZ towels are strongest, she should start studying those paper towels to understand how they are different from others...different in ways that lead to their greater strength. She can measure their thickness. She can look at the paper under a microscope. Do the stronger towels have a tighter weave? Are they made from thicker fibers?
These “Consumer Reports” type of student investigations (“Which brand is best?”) are typically more about engineering than science. To get a better feeling for what science is like, encourage students to look at phenomena in the natural world and ask questions about why they are that way. Why is the Moon sometimes visible in the daytime but never when it is full? How do ants know which way to go to get to a distant food source? Why are plant leaves green, rather than red or blue?
Finally, most teachers have taken a “methods” course. Notice that the word “methods” is plural. Just as there are many ways or methods of teaching, depending on things like your educational philosophy, classroom demographics, and subject area, it should not be surprising to hear that there are also many methods of undertaking a scientific investigation, i.e., different ways of acquiring new knowledge. This is one part of the creativity of science—selecting a method appropriate for the question at hand. And that’s just the beginning of the fun!
Never stop learning. ●
Online Resources
Berkeley’s Understanding Science Resource: https://undsci.berkeley.edu
Hypothesis Article: http://tinyurl.com/no-hypoth
NGSS Science and Engineering Practices: https://ngss.nsta.org/PracticesFull.aspx
Problems with “The Scientific Method:" www.sciencenewsforstudents.org/article/problems-scientific-method
The Myth of the “Scientific Method:” www.herinst.org/envcrisis/science/method/myth.html
The Process of Science: http://aas.org/files/resources/The_Process_of_Science.pdf
Matt Bobrowsky is the lead author of the NSTA Press book series, Phenomenon-Based Learning: Using Physical Science Gadgets & Gizmos. You can let him know if there’s a science concept that you would like to hear more about. Contact him at: DrMatt@msb-science.com



