Focus on Physics
The Photoelectric Effect
The Science Teacher—October 2019 (Volume 87, Issue 3)
By Paul G. Hewitt
It’s commonly thought that Albert Einstein won the 1922 Nobel Prize for his work on relativity. Not true. Einstein’s prize was for his earlier 1905 explanation of the photoelectric effect, a phenomenon later incorporated in devices such as electric eyes, light meters, and, before digital, readers of motion picture soundtracks. Today, the photoelectric effect is applied to photovoltaic electric cells on the roofs of buildings and in sprawling solar energy farms.
Ejected electrons
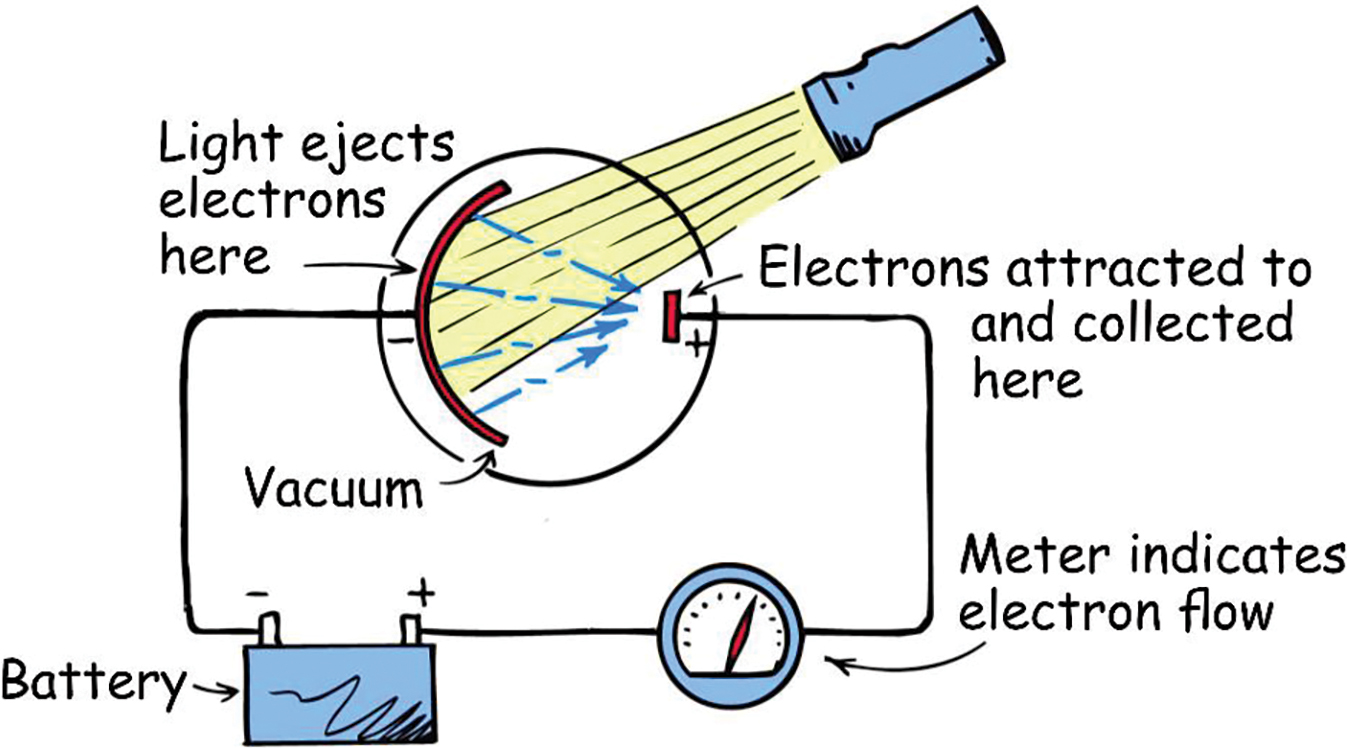
Quite simply, the photoelectric effect is the ejection of electrons from certain surfaces or from atoms below those surfaces when illluminated with light. Several investigators in the latter part of the 19th century noted the curious ability of light striking metal surfaces to create electric current. Figure 1 shows an arrangement for observing this “photoelectric effect.” Light shining on the negatively charged curved metal plate liberates electrons that are attracted to a small positive plate. This process produces a measurable electric current.
Energy of ejected electrons
When the polarity of the plates is reversed, attraction becomes repulsion, and with just enough buildup of negative charge on the plate, the current can be stopped. The easily measured voltage difference between the metal surface and the plate affords a way to calculate the energies of the ejected electrons.
The minimum energy required to eject an electron from a metal’s surface is called the work function of the metal. Any energy beyond the energy used to eject the electron goes to kinetic energy in the electron.
Early investigations
Although Newton had, centuries before, postulated that light was a stream of particles, classical physics at the time of Einstein’s studies held that light traveled in waves, confirmed by the experiments of Thomas Young and others who demonstrated diffraction and interference of light. Electron ejection could be accounted for by classical physics, wherein energy in incident light waves builds an electron’s vibration to greater and greater amplitudes until it finally breaks loose from the metal surface, just as water molecules break loose from the surface of gradually heated water. But no build-up time was observed. None. Depending on the frequency of the light, electrons were either ejected immediately or not at all. Something was amiss.
Incompatible observations
Careful examination of the photoelectric effect led to several observations that weren’t compatible with the classical wave picture:
- The time lag between exposure to light and the ejection of the first electrons was unaffected by the brightness or frequency of the light.
- The effect was seen with violet or ultraviolet light, but not with red light.
- The rate of electron ejection was proportional to the brightness of the light.
- The maximum energy of the ejected electrons was unaffected by the brightness of the light, but it did depend on the frequency of the light.
More troubling observations
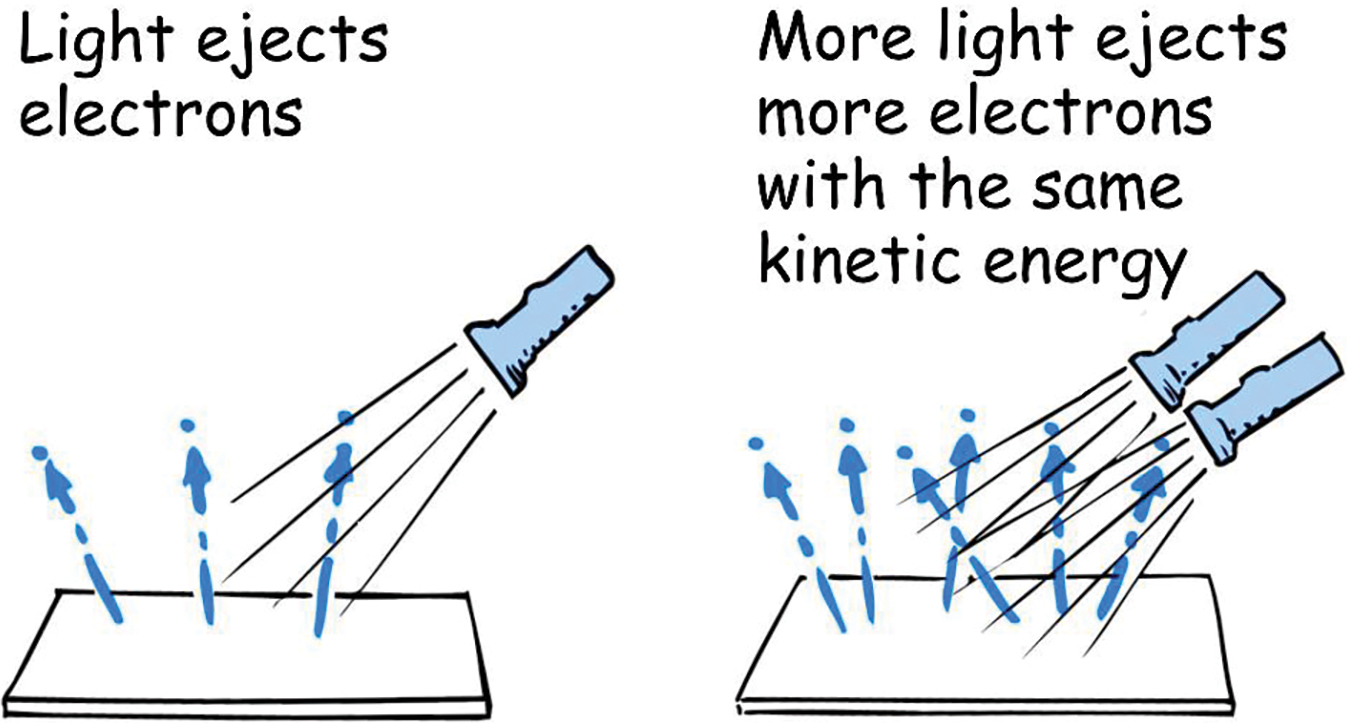
The absense of a time lag was especially difficult to understand in terms of waves. It took practically no time for a weak source of light of the right frequency to impart enough energy to eject electrons from the surface. An electron in dim light should, after some delay, accumulate sufficient vibrational energy for ejection, while an electron in bright light should be ejected almost immediately. But that was not observed. Even dim light caused immediate ejection of some electrons, and the brightness of light had no effect on the energies of the ejected electrons. A brighter light ejected more electrons (Figure 2), but not at greater energies. And a weak beam of ultraviolet light produced a smaller number of ejected electrons but at much higher energies. All of this was perplexing.
A particle view of light
Einstein offered an answer in 1905, the same year he explained Brownian motion and set forth his theory of special relativity. His clue was Max Planck’s quantum theory of radiation. Planck hypothesized that all material systems can only absorb or give off electromagnetic radiation in discrete bundles, which he called quanta. According to Planck, the energy in each bundle of energy is proportional to its frequency of vibration, leading him to conclude that the energy of matter changes by quantum amounts. He hadn’t yet seen what Einstein saw five years later—that light itself comes in quantum bundles. Einstein attributed quantum properties to light itself and viewed radiation as a hail of corpuscles that we now call photons.
E = hf
When the energy of a photon is divided by its wave frequency, a constant value of 6.6 × 10-34 J·s is found; we now call that Planck’s constant and write it as h. The energy of a photon is given by the celebrated equation, E = hf, where E is the energy of the photon, f is the frequency of the light, and h is Planck’s constant.
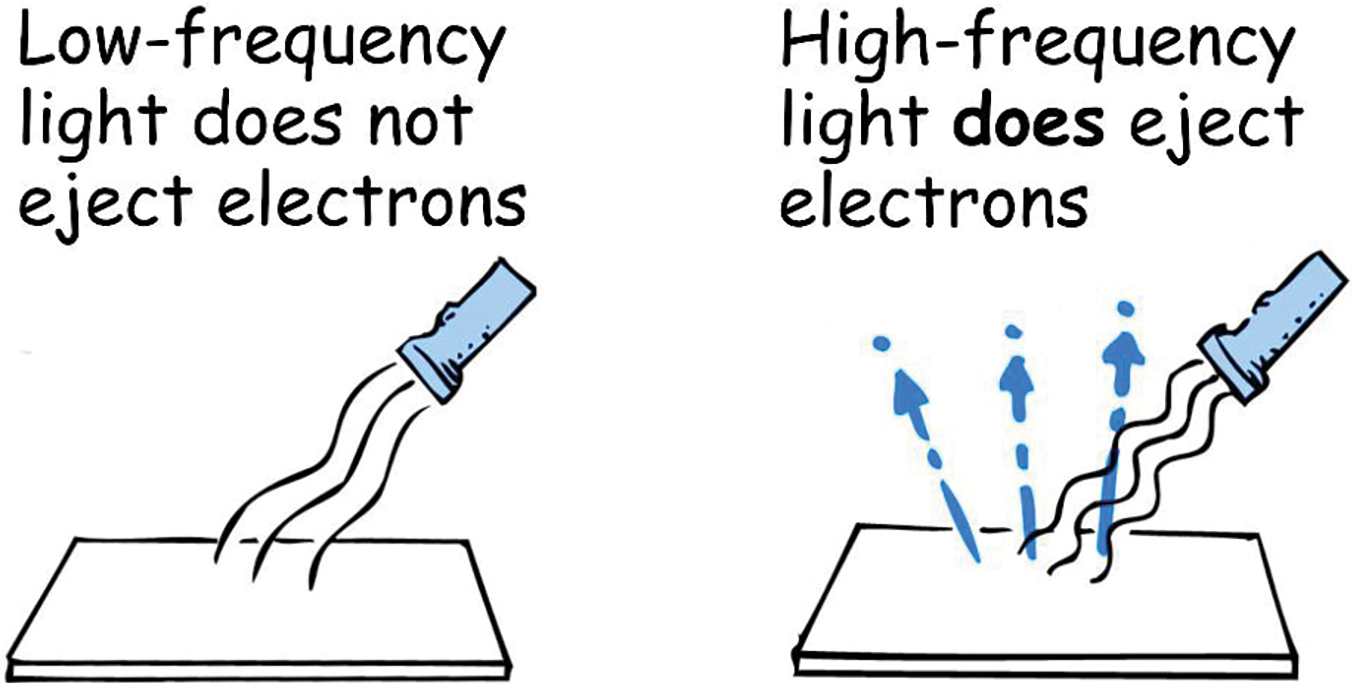
So red light, however bright, is composed of photons of low frequency, and therefore low energy. Violet and ultraviolet light, on the other hand, are composed of higher-frequency-higher-energy photons, all of which explains why ultraviolet light ejected more highly energetic electrons than, say, blue or green light. Red light, however bright, was insufficient to eject electrons from most metals (Figure 3). Einstein postulated that one photon is completely absorbed by each electron ejected from the metal surface. The absorption is an all-or-nothing process and is immediate, so there is no delay as “wave energies” build up.
Although the number of photons in a light beam affects the brightness of the whole beam, the frequency of the light controls the energy of each individual photon. Each photon gives its full energy E to one and only one electron. Each photon, Einstein concluded, throbs at a frequency f and carries an energy hf.
Robert Millikan
Ten years after Einstein’s explanation of the photoelectric effect, all of Einstein’s predictions were verified by the American physicist Robert Millikan in his laboratory. It is interesting to note that Millikan spent a decade trying to disprove Einstein’s theory of the photon. Even after verifying Einstein’s equations in detail and accurately measuring Planck’s constant, Millikan could not bring himself to accept the reality of photons. Nevertheless, he was awarded a Nobel Prize in 1923 for this work. Other work in that same year, by Arthur Compton, finally prompted most physicists to accept the reality of the photon (which didn’t get its present name until 1926—until then it had been a “corpuscle”).
Light as a wavicle
The photoelectric effect demonstrates conclusively that light has particle properties. We cannot conceive of explaining the photoelectric effect on the basis of waves. On the other hand, the phenomenon of interference demonstrates convincingly that light has wave properties. We cannot conceive of explaining interference in terms of particles. In classical physics this is contradictory. From the point of view of quantum physics, light has both properties. Whether light behaves as a wave or a particle depends on the particular experiment. Hence we think of light as both—as a wave– particle. I tell my students to think of light as a “wavicle.” Nature is indifferent to the names we assign to things. More important than any name, quantum physics called for a new way of thinking.
Photovoltaic cells

When you use Sun-generated energy to charge a cell phone, or when you view a small set of solar cells atop a traffic light in rural areas, or a huge array of solar cells on a solar farm, think of Albert Einstein—and how he explained a puzzling relationship between light and ejected electrons in certain materials. The recent advent of photoelectric solar cells is another celebration of nature.


