Science 101
Q: What Is “Static Electricity,” and How Can I See Its Effects?
A: There are many simple ways for students to explore static electricity using simple materials. I’ll describe several fascinating activities using balloons and other items and then provide a brief tutorial about static electricity. You can do each of these investigations as a demonstration or, if your students are up to it, give each group their own materials so that they can do the investigation themselves.
One important tip regarding static electricity activities: Do them only on days when the humidity is low, say, less than 50% relative humidity. On rainy days, or days with high humidity, these activities won’t work as well and might not work at all.
Two Balloons
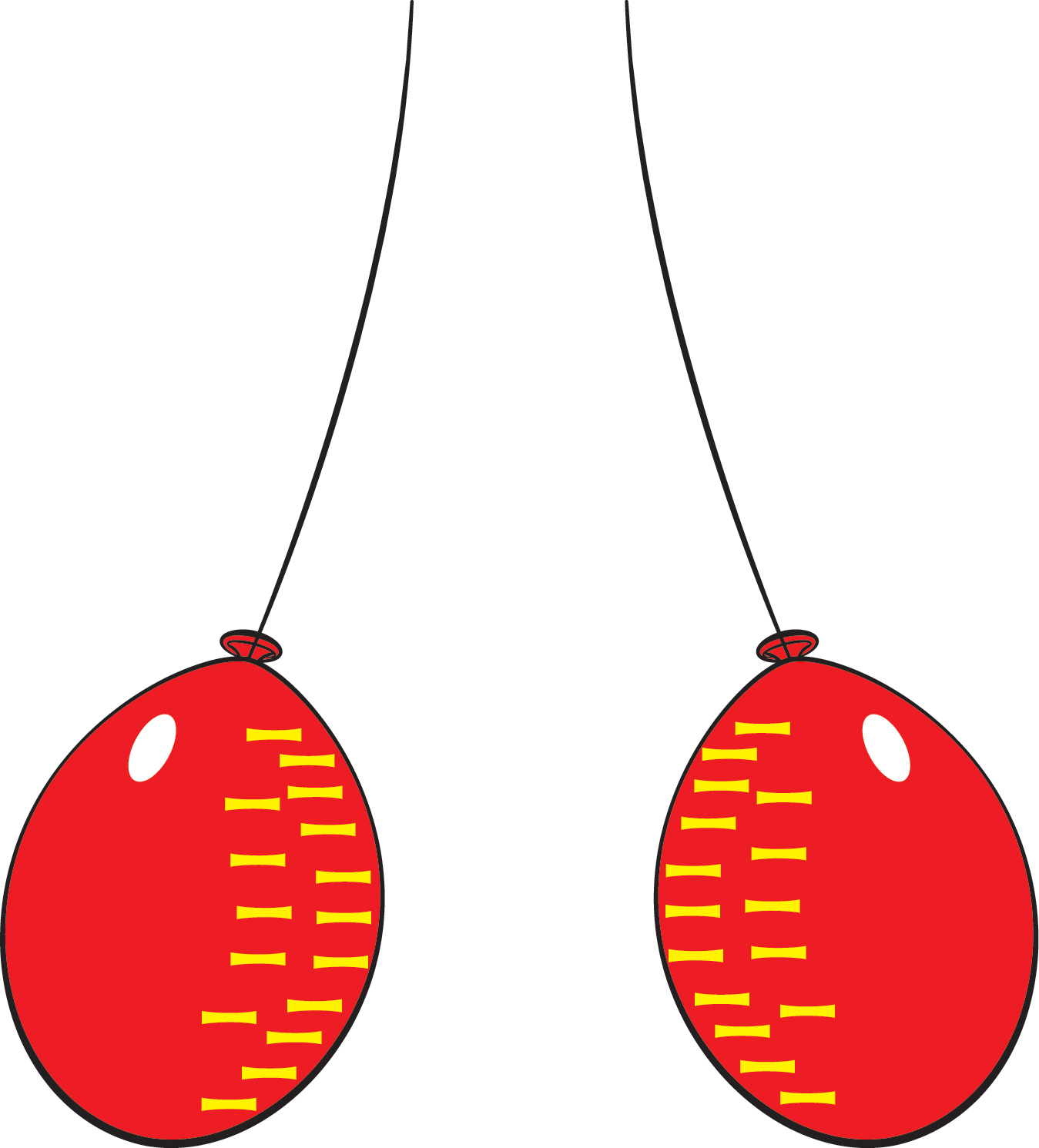
Let’s give two balloons an electric charge and see how they behave. Start with two inflated balloons with strings attached to them.
- Ask two students with long hair to rub the balloons against their hair for at least 20 seconds (or they can rub the balloons against a wool sweater; perhaps let students try both methods and see which works better).
- Hold the strings up so that the balloons hang down near each other (Figure 1).
- Observe what the balloons do (they move apart) and try to formulate a possible explanation. (Explaining phenomena that we observe is what science is all about!)
Additional Background Information
As explained in the tutorial below, rubbing the balloons against hair or wool causes the balloons to become electrically charged. They have the same charge, and like charges repel, so the balloons move apart. After a while, the charged particles leak off, i.e., the excess electrons invisibly flee into the air. Then the balloons are no longer charged, and they move closer together.
Balloon and Hair
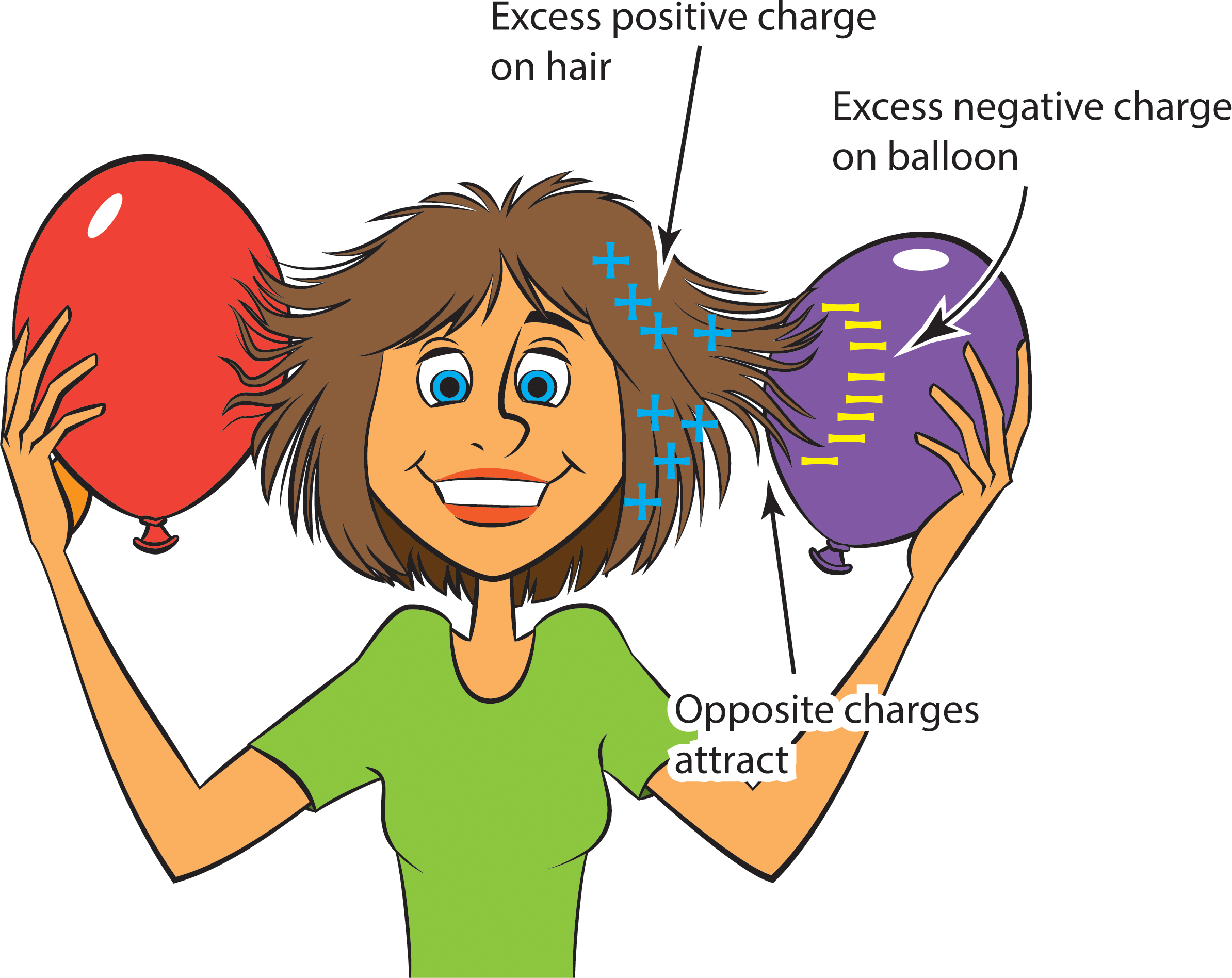
- Have one student in each group rub their hair with an inflated balloon for at least 20 seconds.
- Ask the student to slowly pull the balloon away from their head, while other students observe the interaction between the hair and the balloon.
- Discuss a possible explanation.
Additional Background Information
Rubbing the balloon against hair causes electrons to move from the hair to the balloon. Because electrons are negatively charged, the balloon acquires a negative charge, while the hair, with its loss of negative charges, now has a net positive charge. So the balloon and hair have opposite charges, and opposite charges attract each other. That’s why the hair gets pulled toward the balloon (Figure 2).
Balloon and Soda Can
- Lay an empty soda can on its side.
- Have one student in each group rub a balloon against their hair (or against a wool sweater) for at least 20 seconds.
- The students with balloons should then hold their balloons close to the soda cans (without touching the cans), while all students observe the interaction between the balloon and the can.
- Discuss a possible explanation.
Additional Background Information
Rubbing the balloon against hair or wool causes electrons to move from the hair or wool to the balloon. Because electrons are negatively charged, the balloon acquires a net negative charge. The balloon’s negative charges are attracted to the positive charges in the can, and so the can rolls toward the balloon. As the balloon is pulled away from the can, the can will continue to roll toward the balloon (Figure 3).
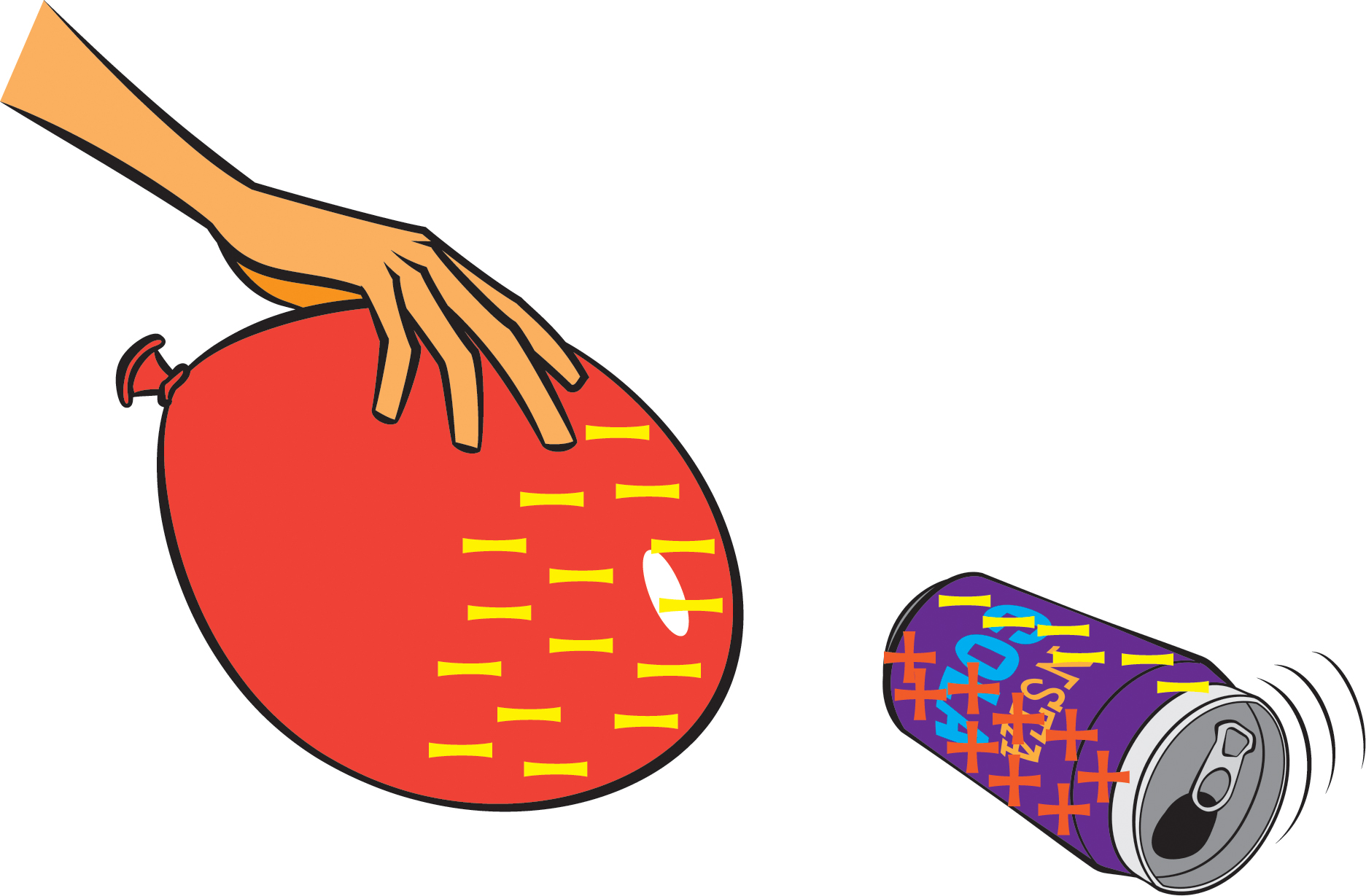
But aren’t there also negative charges in the can, which would be repelled by the negative charges on the balloon? You ask great questions! It turns out that the negative charges (electrons) in the can do get repelled, but because the can is a good electrical conductor, those negative charges simply move to the part of the can away from the balloon, leaving the part of the can near the balloon with a net positive charge, which gets attracted to the negatively charged balloon.
Electrically Charged PVC Pipe
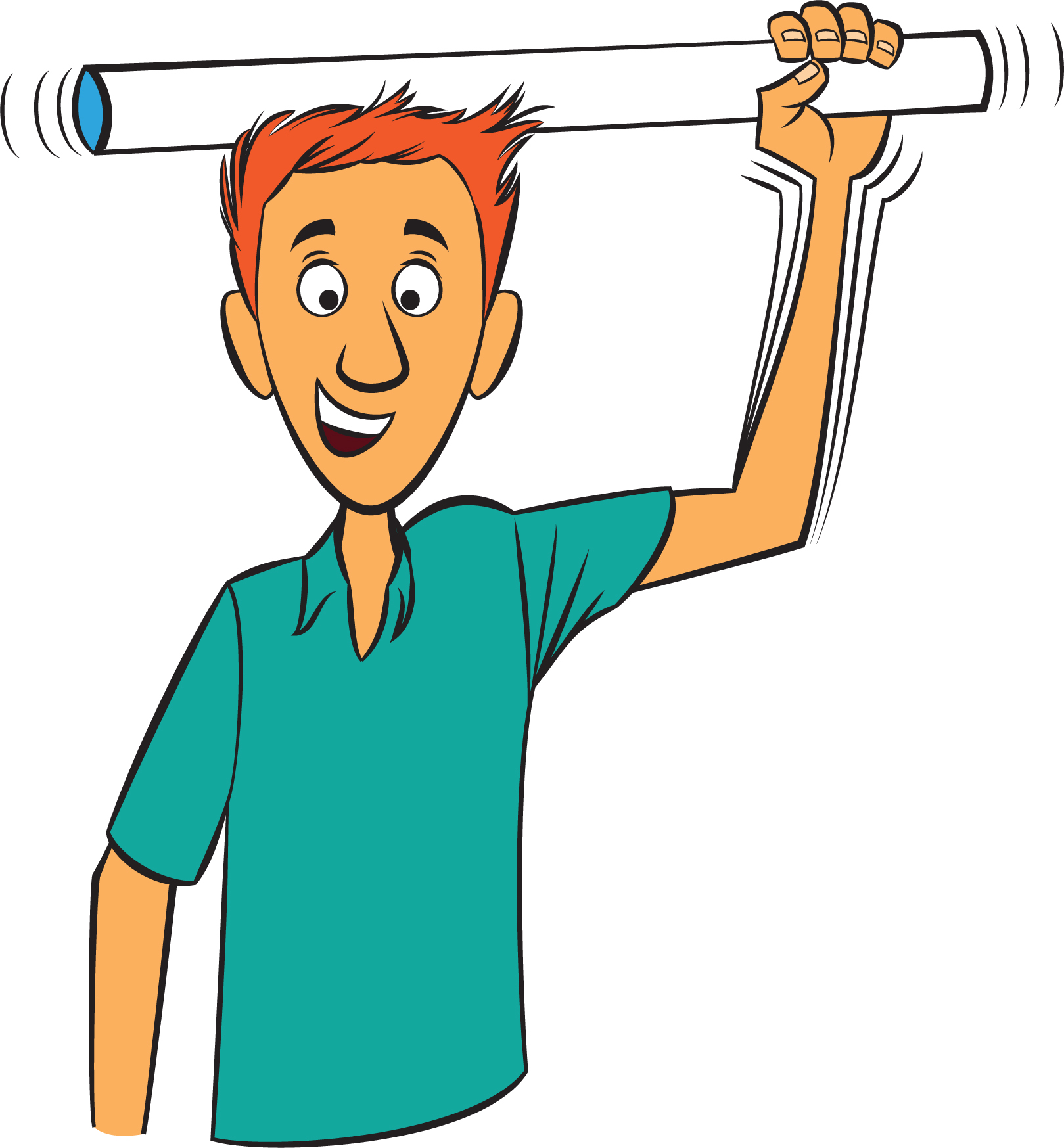
You can make an electrically charged “science wand” by getting a two-foot length of one-inch-wide PVC pipe. Rub the pipe against your hair for at least 20 seconds to charge it up. See what happens when you put the wand near:
- bits of torn-up tissue paper
- some Rice Krispies or puffed rice
- bits of Styrofoam
- thin pieces of tinsel
You or the students will have to recharge the wand every minute or so. You can also try placing a piece of thin tinsel on the charged wand, shake it off, and, moving the wand under the tinsel, keep the tinsel levitated. There is also a gadget you can purchase that does this at the push of a button, called the Fun Fly Stick, which comes with several tinselly shapes that you can levitate due to like charges repelling one another (Figure 4, p. 66). The Fun Fly Stick is available from arborsci.com and other vendors.
Static Electricity Tutorial
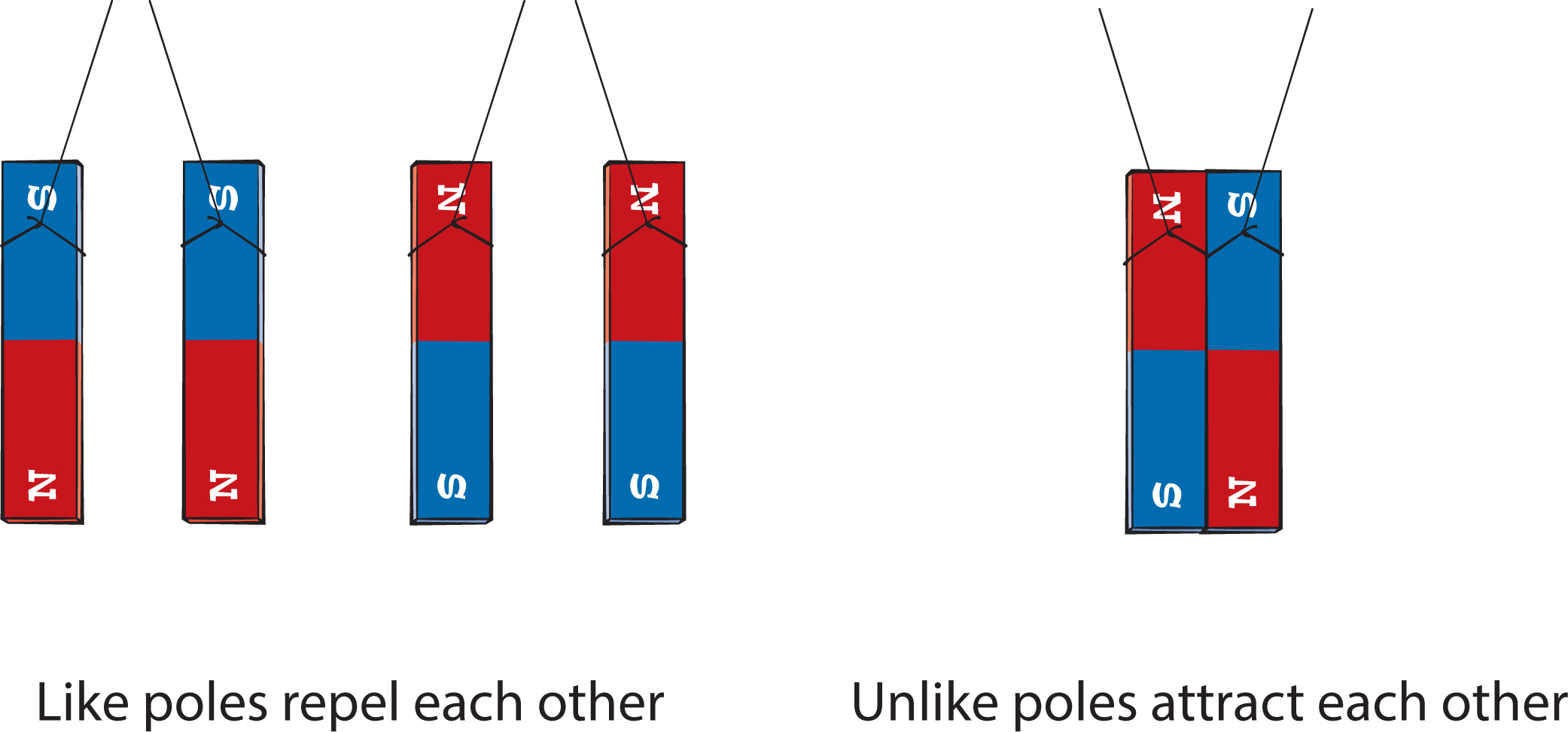
When electricity flows through a wire, what’s actually moving are charged particles called electrons. There are other charged particles in atoms called protons. Electrons and protons have opposite charges, and scientists distinguish the two by labeling an electron’s charge as negative charge and a proton’s charge as positive charge. This is similar to how there are two opposite poles of a magnet. And just as opposite poles of a magnet attract each other, and like poles repel each other, opposite electric charges will attract each other, and like charges will repel each other (Figure 5). Because these attraction and repulsion properties work the same for electric charges as they do for magnetic poles (i.e., opposites attract), you might want to consider reviewing the properties of magnets before investigating static electricity.
When the electric charges move through a wire, you have an electric current, or, simply, electricity. Only the negative charges (electrons) move through a wire. But you can have a buildup of either negative charges or positive charges in an object, and then that object is electrically charged. If those charges aren’t moving anywhere (yet), we say that there is a static charge, or static electricity. Makes sense, right? Personally, I don’t like the term static electricity, because we usually think of electricity as involving an electric current, and in the case of static electricity, there is no current. But static electricity is the popular term, so we use it in the lower grades. In higher grades, we’ll refer to it as an electric charge or an electrostatic charge. You might run into those terms if you read more about this.
A key point is that if an object has more electrons than protons, i.e., more negative charges than positive charges, then the object has an overall negative charge. If an object has more protons than electrons, i.e., more positive charges than negative charges, then it has an overall positive charge. When students rub balloons on a wool sweater or on their hair, electrons get transferred from the wool or the hair to the balloon. So the balloon ends up with a net negative charge, and the sweater or hair, having lost negative charges, gets a net positive charge. And since opposite charges attract, the balloon will stick to the sweater or to someone’s hair after being rubbed on it. If a student with long hair rubs a balloon on her or his head and then slowly pulls the balloon away, students can see the hair drawn toward the balloon. This confirms that the balloon and hair had opposite charges. In fact, any time a balloon sticks to something (assuming you haven’t attached it with sticky tape), it’s because you have opposite charges.
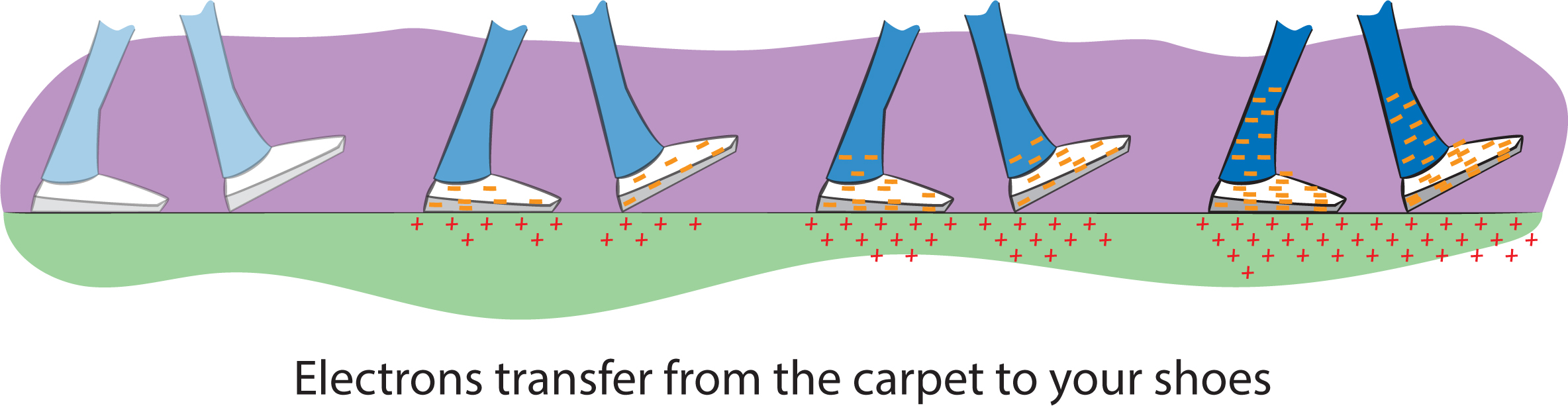
If a charged object touches another object, especially a good conductor like metal, the charges can suddenly scoot out of the object, leaving it with no charge. Your students have probably experienced walking on a carpet (especially if they’re carelessly dragging their feet) and then getting a shock when touching a doorknob. If they walked on a wool carpet with rubber-soled shoes, electrons were transferred from the carpet to their shoes (and bodies), so they built up a negative charge (Figure 6). When touching the doorknob, the charges suddenly jumped to the metal, creating the feeling of getting shocked. Now you know why you have such an electrifying personality!
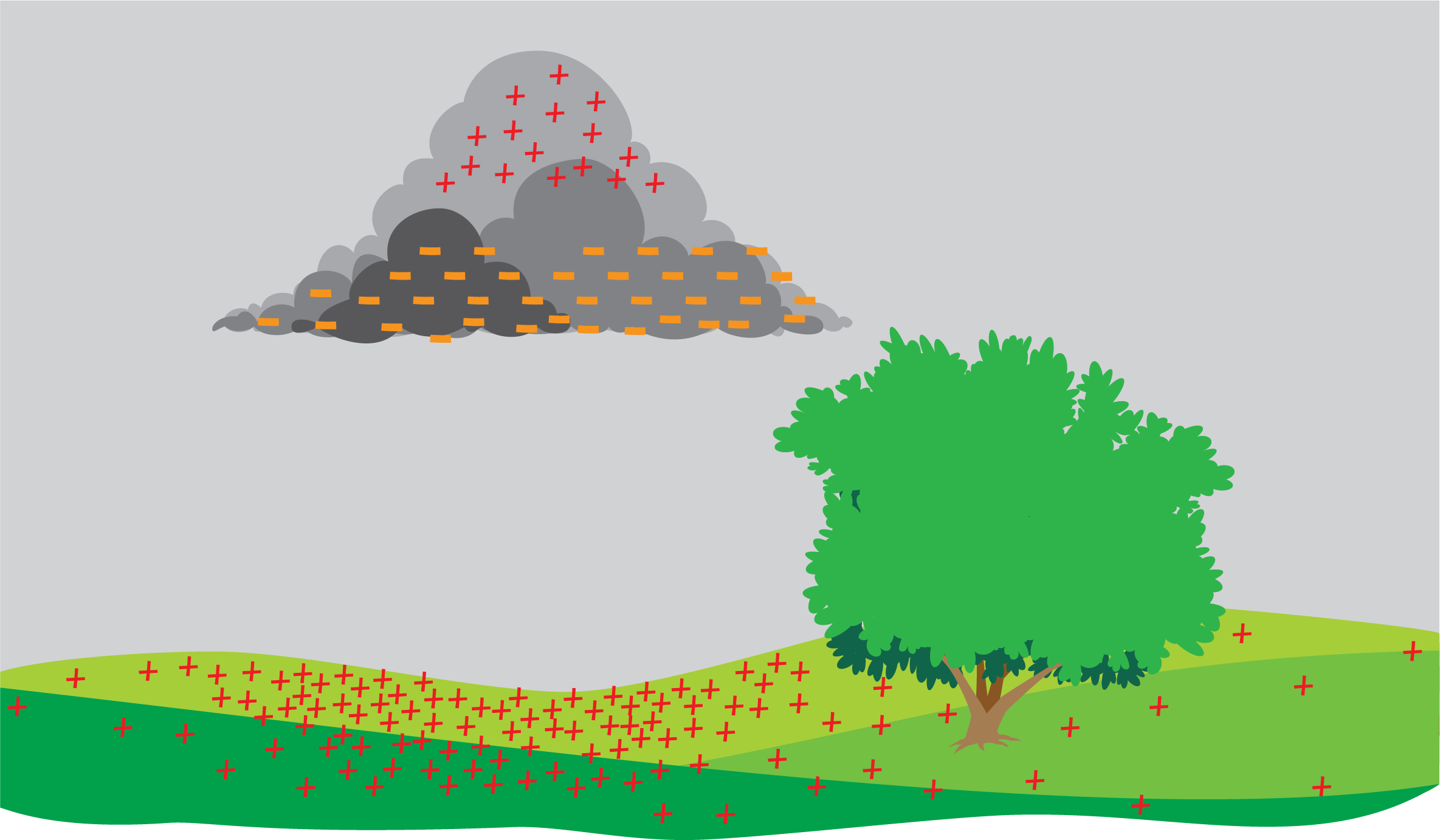
Fun fact: That little spark you get when you touch the doorknob is essentially a miniature lightning bolt. When rain clouds move through the air, they build up positive and negative electric charges (Figure 7). So clouds have static electricity too! And when enough charge builds up, electric charges jump between the cloud and the ground. That’s the spark we call lightning! Wasn’t that enlightening?
Never stop learning!
Instructional Materials Phenomena Physical Science Elementary



