Science 101
What’s a Fun Activity That Combines Science With Art?
Science and Children—July/August 2020 (Volume 57, Issue 9)
By Matthew Bobrowsky

“George won’t eat anything green. So, I have to dye it.”
A: There are many connections between science and art, and one of the easiest to investigate—in school or at home—has to do with color. Here’s a quick and easy investigation:
Have the kiddos arrange some M&M’s in a circle on a plate, as shown in Figure 1. Then slowly pour some water in the middle of the plate until there’s enough water that the M&M’s are in the water but not so much water that it extends beyond the M&M’s. During the few minutes it takes for something to happen, let’s talk about the relevant science. Water’s ability to break things down and cause them to become part of, or dissolved in, water is called solubility. A liquid that can dissolve other substances is called a solvent. You can stir some grains of salt or sugar into warm water and watch those grains dissolve and disappear. They broke down into particles too small for you to see.
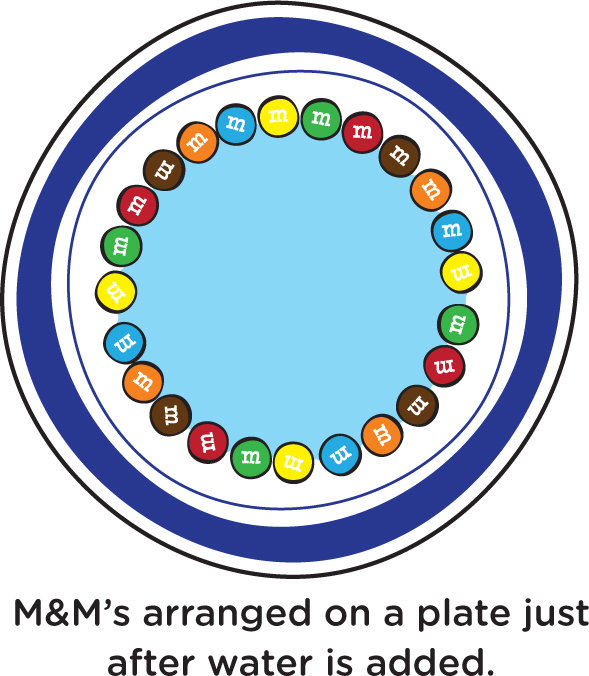
The pigments, or colored dyes, on the M&M’s are soluble in water, meaning that they will dissolve in water (or in your mouth, where there is water). After a few minutes, what do you see? Some of the colored dyes have dissolved in the water, and the longer you wait, the farther you see the colors have spread out from the M&M’s (see Figure 2). It looks beautiful, like a work of art, but it’s entirely a result of well-understood science.
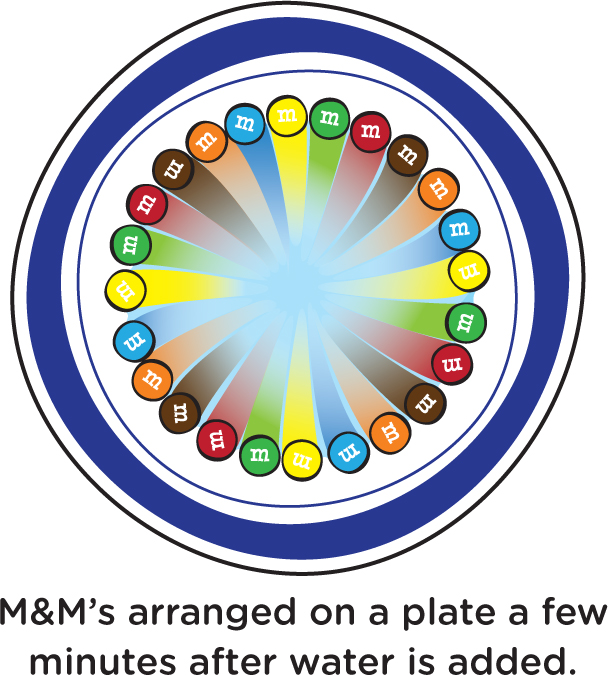
It turns out that different colored molecules will move, or diffuse, through the water at different speeds. And this fact allows scientists—and the future scientists with whom you are doing these investigations—to see how sometimes there are different colors in a substance that aren’t initially visible. Let’s try an interesting activity that will show an example of this.
You’ll need one or more black markers, such as Crayola, EXPO Vis-à-Vis, Flair, and/or Cra-Z-Art markers. (Don’t use a Sharpie; this activity will not work with a Sharpie.) By using more than one brand of marker, your young investigators will see one way that the various brands of markers differ from one another.
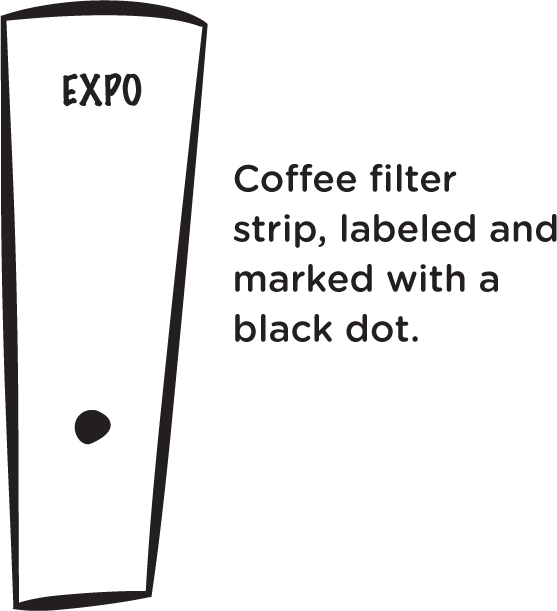
Take a white coffee filter and cut it into four inch strips, about an inch wide. You’ll need one strip for each kind of marker that you’ll be testing. With a pencil, write the brand name at the top of a strip, and, with the marker, make a pea-sized, black dot on the strip, about 1.5” (two finger widths) from the bottom (see Figure 3). Do this on each coffee filter strip for each different brand.
Get a small bowl and put about one inch of water in it. Then dip the bottom of the strip of paper in the water, but don’t put the black dot in the water (see Figure 4, left). Hold it there for a couple of minutes—until water has soaked up far enough so that you can lay the strip over the edge of the bowl and have it stay there. Repeat for each strip and marker (see Figure 4, right).
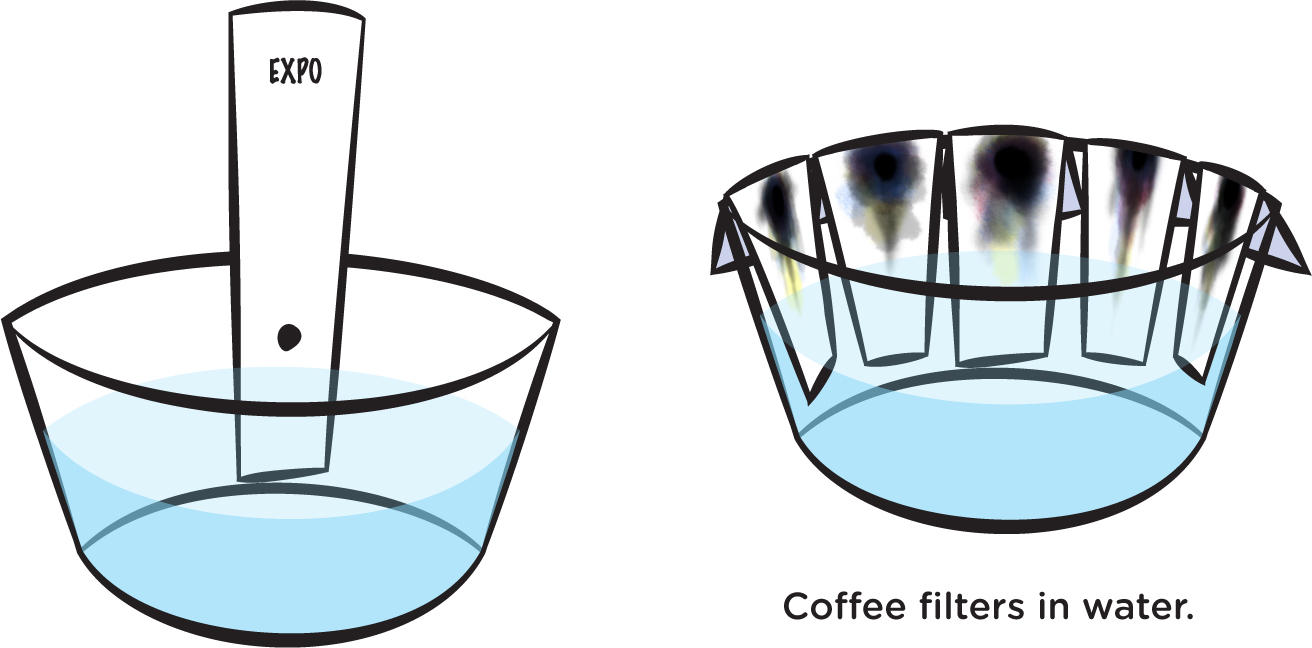
When the water has soaked up almost to the top, take the strips out of the bowl and lay them flat to dry on a paper towel.
Observe the different colors! Did you know that the black ink contains all those colors?
You can ask your young experimenters questions like these: What colors make up the black ink? Were the colors different for the different brands of markers you tested? In what way were they different? What is the solvent? (water) What was dissolved in the water? (the dyes or pigments in the ink)
What’s Going On?
The black ink in a marker is a mixture of different substances having different properties—different colors, different size molecules, and so on. As the water travels through the filter paper, the water acts as a solvent and dissolves the various components of the mixture, so these components (having different colors) travel through the water up the filter paper. Because of the different properties of the various dyes, they will travel at different speeds and separate. This is just like when some people are having a running race, and they all start at the same place, but as the race goes on, different runners move at different speeds and therefore separate from each other.
Why do the different colors move at different speeds? Because the molecules that make up those different colors have different sizes, and the smaller particles are able to move through the water faster than the larger particles. Now that you know that, let’s look at the colors on one of the paper strips (see Figure 5) and see if you can determine which colors are made of smaller molecules, and which colors are made of bigger molecules. Think about that before reading on.

It looks like some of the red pigment is still very far down on the paper. Those molecules must be the biggest ones, causing them to move the slowest. A bit higher, we see some yellow, which must be made of slightly smaller molecules. At the top we see the blue pigment. Since the molecules in the blue pigment moved the fastest, they must be the smallest. Isn’t it amazing that, from such a simple procedure, we can tell which molecules are smaller or bigger than others? I think so!
Why Not a Sharpie?
Remember I said that this wouldn’t work with a Sharpie? The reason is that the ink in a Sharpie does not dissolve in water. (That’s what makes it a permanent marker.) You can still do this activity with a Sharpie, but you’ll need to use a different solvent. You can try it with alcohol (while wearing safety goggles). If you do, I’d be interested in hearing what kind of results you get.
Why Does the Water Travel up the Filter Paper?
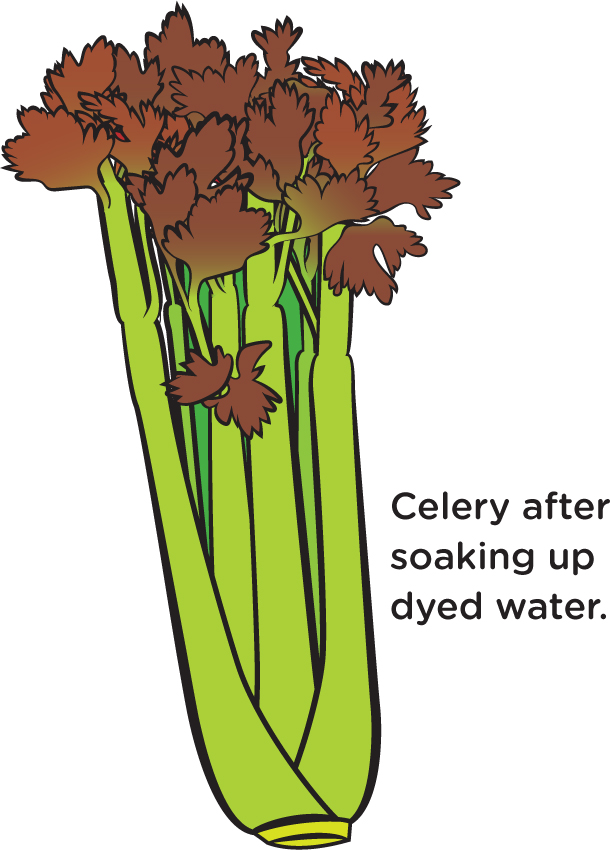
The water travels up the filter paper due to capillary action. Capillary action occurs when water moves up something solid, like a thin tube, or in a porous material, i.e., one with many small holes, like your coffee filter paper. Why does it do that? Basically, because water molecules stick to things. You might not think of water as being sticky in the same way that Scotch tape is sticky, but water molecules are attracted to one another, and they are also attracted to the fibers in the filter paper. So, some water molecules touch some of the fibers a little higher up and stick to them. Then those water molecules pull up other water molecules. And the process keeps repeating, as the water pulls itself higher and higher. This happens in plants, as capillary action results in water traveling up the thin tubes in the stem or stalk. You can see this by placing a celery stalk in water. Color the water with food coloring and after a number of hours, or overnight, see (as in Figure 6) how high the dye has risen.
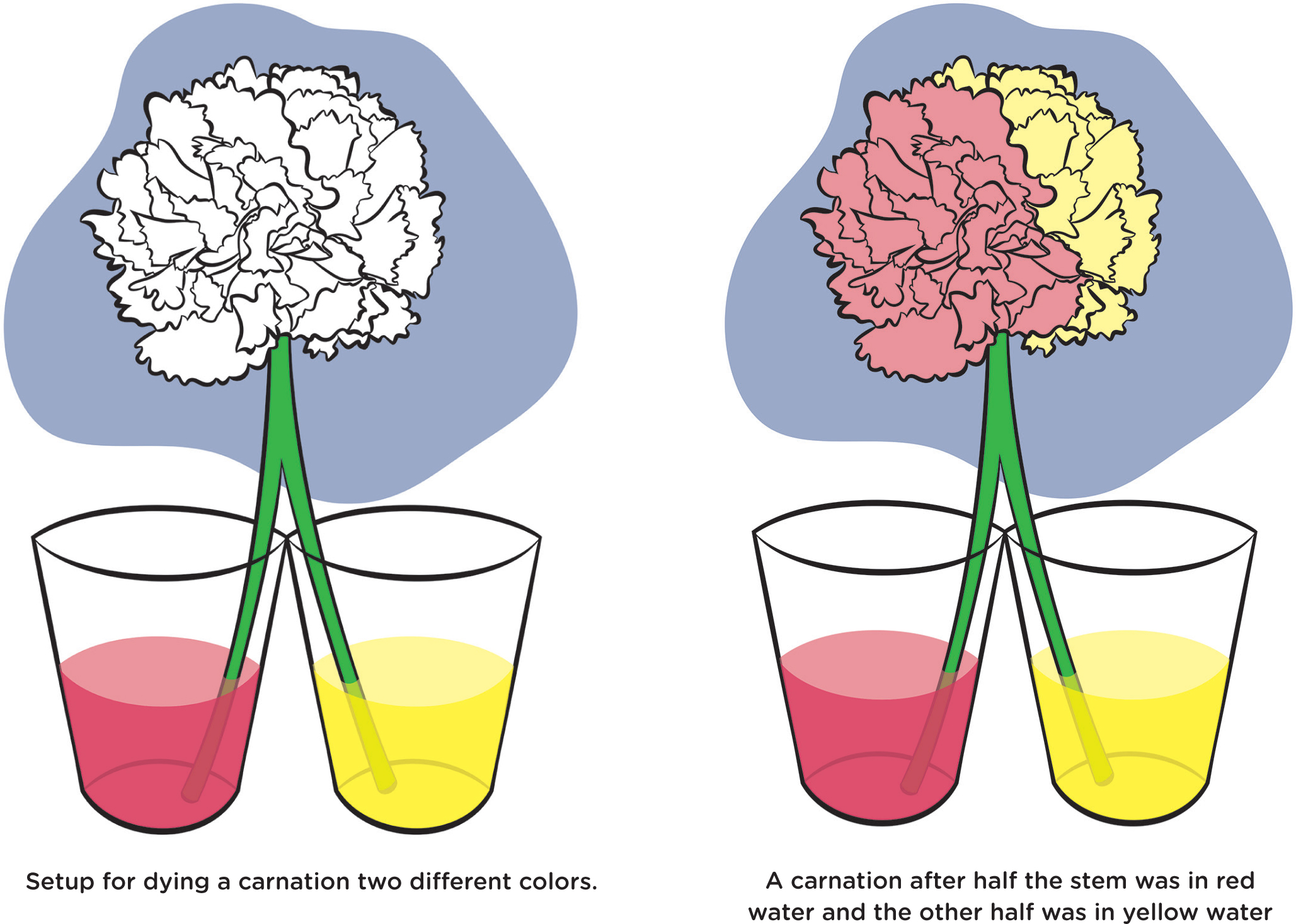
In the same way, you can color white carnations. Trim the bottom of the stem, and then place the stem in dyed water. The color will migrate via capillary action all the way to the flower petals. You can even make it more than one color by carefully pulling the stem apart at the bottom, and putting each part of the stem in a separate cup of water with a different color dye in each cup (see Figure 7).
Think about a time when you spilled something on your kitchen counter or on the floor. You grabbed a paper towel and put it on the spill, and the water immediately soaked into the paper towel. You used capillary action to clean up your mess!
We’ve connected science, art, and cleaning up spills. Science is everywhere! Never stop learning.
What’s the Good Word?
A procedure like this, which can separate the differently colored components of a mixture, is called chromatography. The first part of this word, chromato-, means that it has something to do with color, as in the word chromatic. And the second part of the word, -graphy, means making a record of something, as in photography. In this case, you’ve made a record of how easily or fast the different colored pigments travel through water. And the particular kind of chromatography that you employed is called paper chromatography. You can Google that for more information.
Matt Bobrowsky is the lead author of the NSTA Press book series, Phenomenon-Based Learning: Using Physical Science Gadgets & Gizmos. You can let him know if there’s a science concept that you would like to hear more about. Contact him at: DrMatt@msb-science.com


