FOCUS ON PHYSICS
Crunching Cans and Generating Power
The Science Teacher—July/August 2021 (Volume 88, Issue 6)
By Paul G. Hewitt
We give little or no thought to the tremendous force produced by the atmospheric pressure continually exerted on us. We have learned, for example, that atmospheric pressure on the outside of a classroom window produces tons of force directed inward. And we have learned why the window doesn’t shatter—because the same amount of force pushes outward on the interior surface. Atmosphere pressure on such common surfaces produces no net force. Inside and outside atmospheric forces balance.
Crunching can demonstration
Just like the classroom window, an open container of air is unaffected by atmospheric pressure because the pressure exerted on both the inner and outer surfaces of the container balance one another. Balanced pressures, small or large, produce no net force on an object. But what happens when the push-back force on one side of a surface is reduced, or removed? Dan Johnson illustrates this in Figure 1. He puts a small amount of water in an open gallon-sized steel can. Then he places the can on a hot plate. The water inside turns to steam that can be clearly seen as a cloud of condensed water droplets coming out of the can opening. After much of the steam and air inside is driven out, he seals the can by screwing its cap on its opening and removes the can from the heat source. He and his class watch and hear in awe as the can cools and slowly crumples.

Dan leads his class into an explanation for the can’s crunch. After much of the heated air is emptied, as clearly shown by condensed steam exiting the can, he seals the can air-tight: less air occupies the inside of the can than before heating. As the can and air inside it cools, internal pressure correspondingly decreases. Push-back force inside the can is reduced while pressure outside the can doesn’t change. Now every square centimeter of the can experiences a greater force inward than outward. The can collapses.

Witnessing the collapse is intriguing—understanding the cause of the can’s collapse can be joyful. Joy comes with the personal discovery of an understanding that exceeds what was expected. Aha! The satisfaction of true learning is wonderful.

Crunching drum demonstration
What occurs for Dan’s small can also happens to a larger container. Quite dramatic is watching a 55-gallon drum crumple when air inside is removed by a vacuum pump. Again, atmospheric pressure is central to understanding. Before the drum is partially evacuated, air pressure inside the drum is balanced by the air pressure outside. Air provides no net inward or outward push on the drum. With much of the internal air withdrawn, resulting in little air pressure remaining on the inside surface of the drum, the atmospheric pressure against the exterior does its thing—the net force of the atmosphere on each square centimeter of the drum produces crunching. Watching this demo is dramatic, and not to be forgotten.
Crunching soda-pop can demonstration
The crunches discussed thus far have been relatively slow ones. A quicker one is easier to demonstrate—crunching aluminum soda-pop cans. Place a small amount of water in an empty aluminum soda-pop can and heat it on a stove or hot plate until steam issues from the opening (Figure 2). Air has been driven out of the can and replaced by steam. Then, with a pair of tongs, quickly invert the can into a pan of water. Whop! The can is quickly crunched by atmospheric pressure!
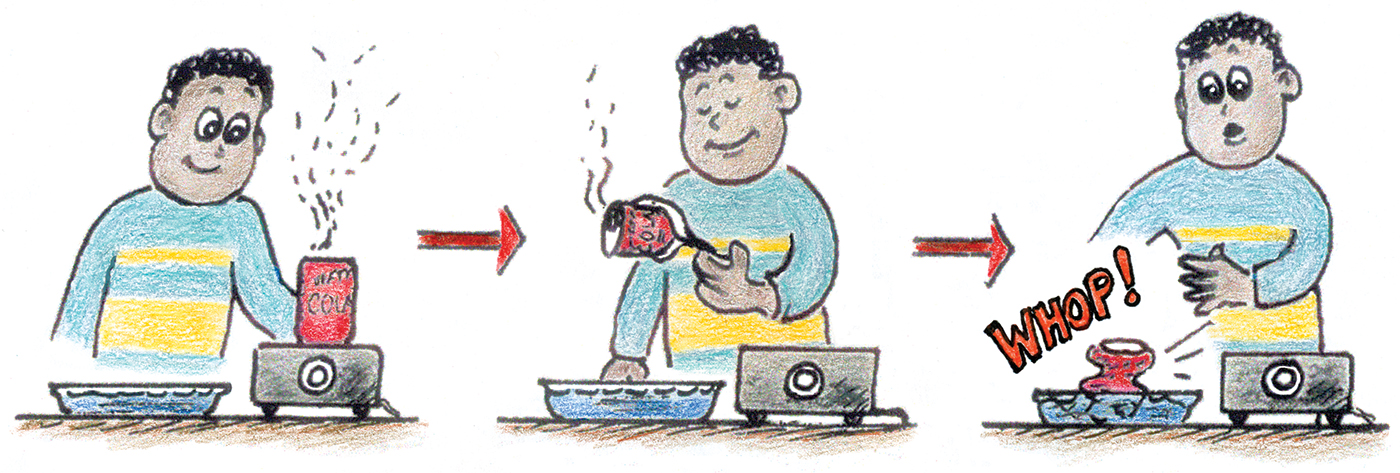
Guide your class through an unhurried discussion of the explanation: Before the can is inverted, steam molecules inside fly around, bouncing from each other and off the walls of the can with little or no net energy loss. The inner metal surface certainly doesn’t absorb them. Although steam molecules that encounter the wall just bounce, they are “swallowed” when they encounter the water in the pan. The water surface needn’t be cold, just wet! Steam molecules immediately stick to the water surface, which is to say that they undergo condensation.
The condensation is quick and accompanied by a rapid decrease of pressure in the can. What does the outside atmospheric pressure do to the can? That’s right. Atmospheric pressure crushes the can.
Turbine power generation
The beauty of the soda-pop can crunch is that it explains a related phenomenon—the use of condensation in a power-generating turbine. For years this author had been stymied trying to understand why condensation was part of the steam cycle of a steam-powered power plant. A steam cycle is shown in Figure 3.
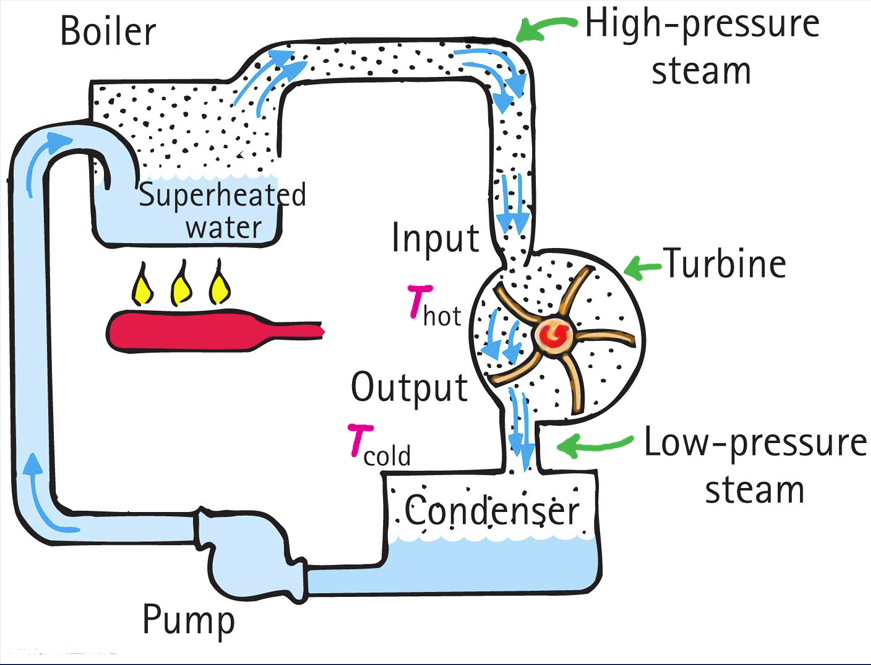
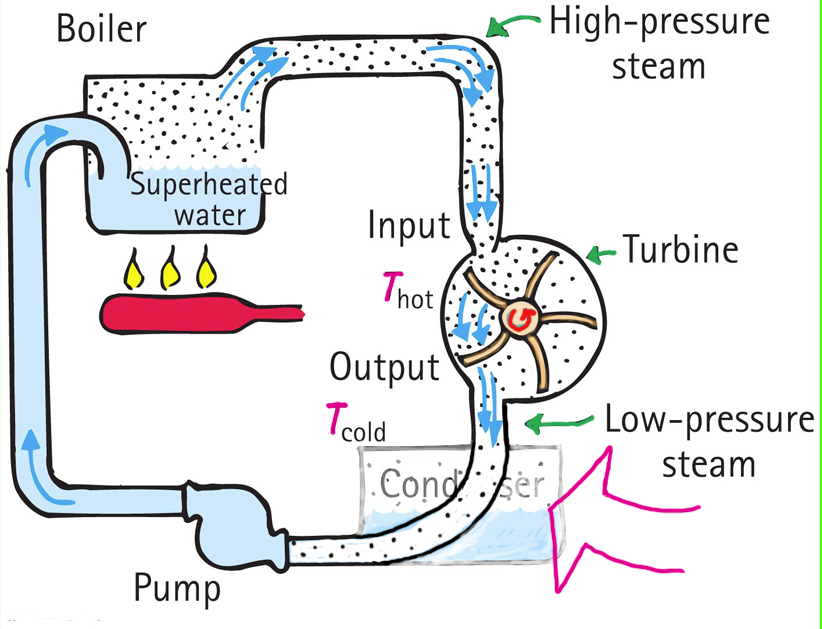
We see that water is superheated and turns into steam. Next, the steam spins a turbine to drive an electrical generator. Finally, the steam is condensed to water that is pumped to its origin for reheating. For years I wondered why the condenser? Isn’t the condenser a waste? We cool the steam down, change it to water, then return it to the boiler and heat it up again. Something wasn’t right here. Why not bypass the condenser altogether and simply send the spent steam directly back to the boiler (Figure 4)? What physics was I missing?
Increasing efficiency
While guest teaching in Hawaii, I joined a small group invited to visit the local steam-powered power plant. I shared with engineers my burning question: Why not bypass the condensation in the cycle? The answer given was that the condensation improves the efficiency of the system. But how, I wondered. Coincidentally, this occurred at a time when I showed the soda-pop crunch demonstration to my class. Voila! I saw the meaningful connection between the rapid drop in pressure in the can due to condensation, and the means of reducing back pressure in a turbine. A big “aha!” moment for me! Very big!
How efficiency is increased
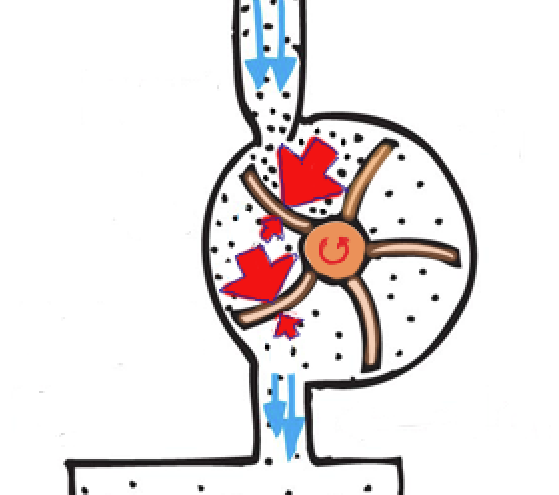
Let’s explore how efficiency is increased by the condenser in the steam cycle in detail. First, we know that electric energy is generated when a steam-driven turbine spins an electric generator. We also know that the turbine operates by differences in steam pressure. Note in Figure 5 that a greater net force by steam on each rotor blade is achieved by greater pressure on the front of each blade than on the back side. This net force produces the net torque that drives the turbine. Without the net torque produced by differences in pressure, the turbine wouldn’t rotate to supply energy to the generator. Hence the importance of reduced pressure on the backside of the rotor blades. How to reduce that pressure? By the same way pressure was reduced to near-zero in our soda-pop can. By condensation!
Essence
Will your students remember these three can-crushing events long after taking your class? Some will, some won’t. If their thinking led to their own discovery of the causes of what they saw, there will more likely be “aha!” moments that will remain in their minds. Teacher skills at invoking such “aha!” moments are priceless.
More important than your students remembering details of your demos is their awareness that physical reasons account for these and other events—their worldview through the lens of science. Unlike their uneducated ancestors who saw the world through magic and superstition, your students see reason rather than mystery. They perceive nature following well-understood rules, central among which are what your course is about—the laws of physics.
After all the details of your course are forgotten, what will remain is its flavor. And that flavor is the knowledge that everything in our physical surroundings is in accord with the laws of physics. A study of physics, after all, is a study of nature’s rules.
On the Web
See related student tutorial screencasts 71 – 81 on heat at www.HewittDrewIt.com, and on www.ConceptualAcademy.com.
Reference
Hewitt, P. 2017. The ever present atmospheric pressure. The Science Teacher 84 (7): 11–13.
Paul G. Hewitt (pghewitt@aol.com) is the author of the new 50-year celebration 13th edition of Conceptual Physics; the 6th edition of Conceptual Physical Science, coauthored with daughter Leslie Hewitt and nephew John Suchocki; and Conceptual Integrated Science, 3rd edition, with coauthors Suzanne Lyons, John Suchocki, and Jennifer Yeh.
Physics Teaching Strategies High School


