Idea Bank
Arguing About a Chemical Change
The Science Teacher—November/December 2019 (Volume 87, Issue 4)
By Patrick Brown
Use a sample ACT writing prompt in an explore-before-explain instructional sequence to a 9th-grade physical science class to promote student learning and demonstrate that mass is conserved in a chemical reaction
Balancing literacy instruction with students’ firsthand science experiences with data and evidence can be a meaningful way to integrate science and argumentative writing. I use argumentative science essays as a tool for prompting deep conceptual thinking while teaching students how to write effective essays. When I first started using writing in science, I expected students to be able to easily explain their ideas and support them with evidence. However, students often have trouble articulating their understanding through writing. When I provided guidance and broke down argumentative essays into smaller parts, students learned to write in more complex ways, while also demonstrating that they learned difficult science content. Consequently, a student’s science achievement and writing ability increased. While argumentative writing may take time to master, the skills developed are worth the trade-off and can help students in science class, on high-stakes tests like the ACT, and in life.
This lesson addresses the Next Generation Science Standards (NGSS) performance expectation (PE) that students can “use mathematical representations to support the claim that mass is conserved during a chemical reaction” (NGSS Lead States 2013; See NGSS table on page 24).Students have trouble distinguishing between chemical and physical changes, as well as difficulty determining when a substance is conserved during a change (Driver et al. 1994). I use a sample ACT writing prompt in an explore-before-explain instructional sequence to a 9th-grade physical science class to promote student learning and demonstrate that mass is conserved in a chemical reaction. The lesson includes a writing task, a demonstration, student evidence-based claims, an authoritative explanation, and student evaluation.
Engage
The purpose of the Engage phase is to situate learning in a meaningful phenomenon while also tapping into student’s prior knowledge and experiences. We start with a writing prompt (see Figure 1). I discuss the essay tasks and scoring criteria in order to promote backward design and start with a clear understanding of our learning goals (Wiggins and McTighe 2005) (see Table 1).
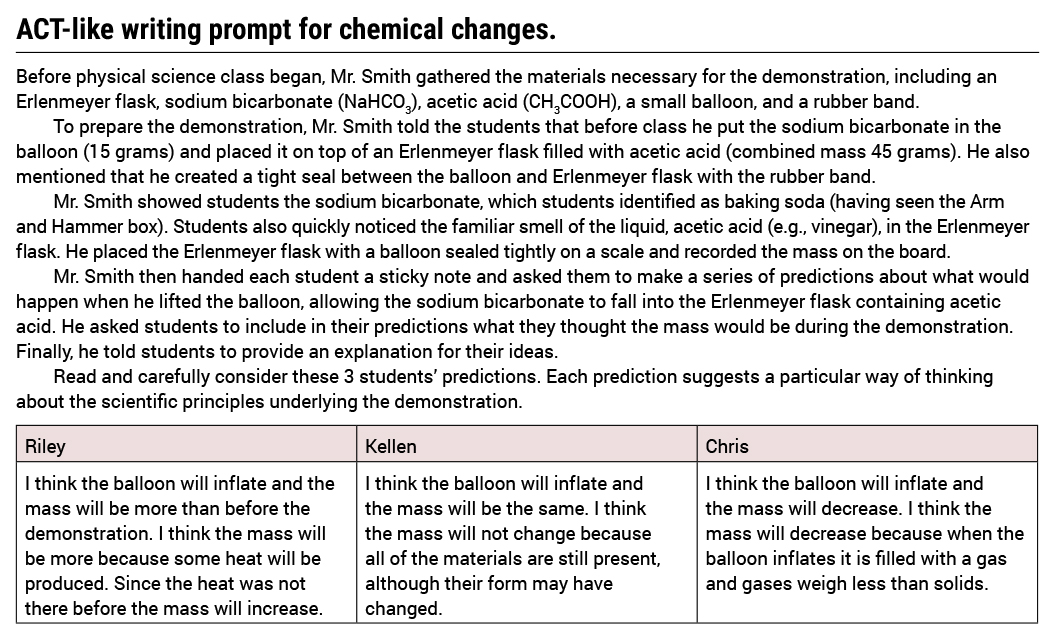
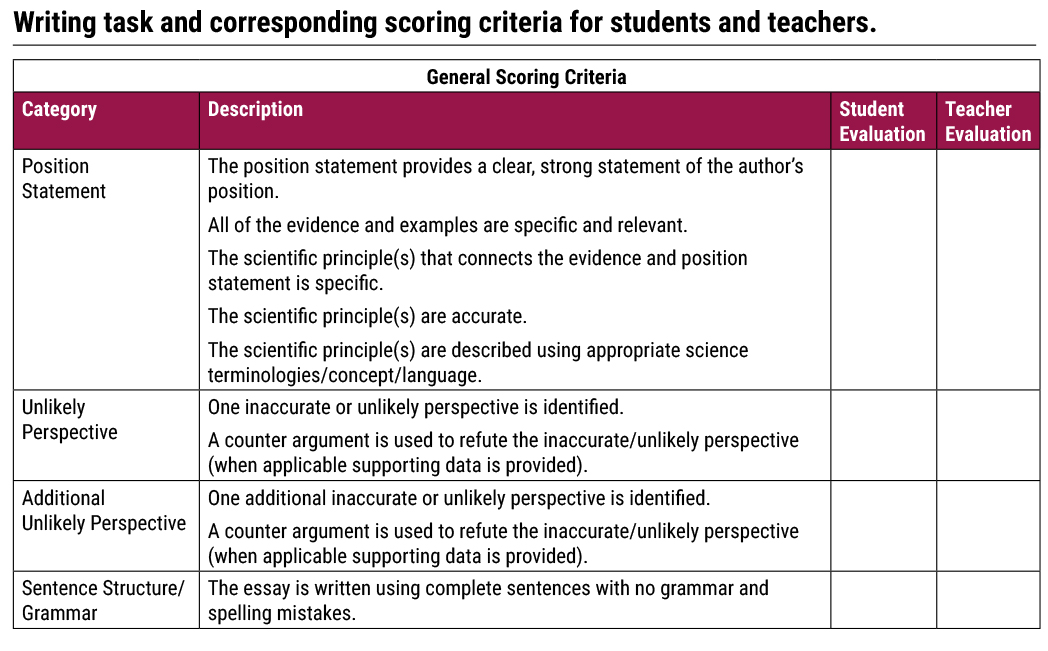
Once students understand the learning expectations and the task, it is time to begin. We start with a close reading of each unique perspective (see Figure 1). I ask students to evaluate Riley’s perspective in terms of the following guiding questions:
What insights do they offer and what do they fail to consider?
Why might they be persuasive to others?
Finally, I ask students to jot some notes indicating their stance on the strength and weakness of the perspective. Once students are finished evaluating Riley’s perspective, they share ideas with a partner. Students move on to the other two student perspectives (Kellen and Chris) using the same guiding questions and shoulder talk with a partner.
The close reading activities highlight key ideas and details in the Common Core State Standards (CCSS) for English Language Arts related to technical texts that ask students to “determine a central idea of a text and analyze its development over the course of the text, including how it emerges and is shaped and refined by specific details; provide an objective summary of the text” (NGAC and CCSSO 2010; CCSS.ELA-LITERACY.RI.9-10.2).
Following student conversations and notes about each perspective, I realized that they did not have a firm understanding of the chemical reaction described in the writing prompt. I heard every possible explanation, from the mass and temperature is less, the same, to more after the reaction. The reasoning provided in each perspective made logical sense to some students, and were used to support some of the explanations. Interestingly, many students mentioned that they had seen the chemical reaction before in other science classes. Student comments about past experiences and incomplete conceptions made me think they needed more opportunities to construct knowledge from firsthand experiences with data.
Research shows that traditional hands-on sequences that these students encountered in previous classes were insufficient in promoting long-lasting understanding (Hofstein and Lunetta 2004). Hands-on activities alone are insufficient, and students need minds-on experiences where they can make sense of data and make scientific claims supported by evidence.
Explore
To delve into whether mass and temperature change during the reaction described in the writing prompt, we needed to conduct further testing. To investigate changes in mass, I put 3 ml of vinegar in an Erlenmeyer flask. Next, I put 3 g baking soda in a balloon. I carefully put the balloon on the Erlenmeyer flask, making sure not to mix the baking soda and vinegar. I use some black electrical tape to seal the balloon to the Erlenmeyer flask, and make sure there is little to no gap.
Finally, I place the flask-balloon setup on an electronic balance. (Note to teachers: This demonstration is challenging to set up and mimics a closed system. Teachers should practice the demonstration beforehand, because if the system is not as closed as possible, gas will be released, promoting misconceptions about conservation of mass). I carefully lift the balloon, allowing the baking soda to drop into the vinegar. Safety note: teachers should wear protective goggles, a heavy-duty apron, and gloves. Although the balloon inflates, the overall mass remains 26.6 g (See “On the web” for a video demonstration).
The next demonstration is designed to test whether a change in temperature occurs during a chemical reaction. Equal amounts of baking soda and vinegar are placed in beakers (I use approximately 33.6 g; however, any equivalent amount would work. Also, I normally would not measure the mass of a liquid and would use volume; however, I want students to know that we are using the same amount of each substance). Next, we record the temperature of each solution; the baking soda was 22°C and the vinegar was 21.9°C. When the two are mixed, students were surprised to see the temperature decrease to 17.6°C (see “On the web” for a video demonstration).
Evidence-Based Claims
At this point in the lesson, I want students to articulate their understanding through writing. I ask students to make a claims-evidence statement; in other words, use empirical data to construct a conceptual claim about the phenomena (the temperature decreased, but the mass stayed the same in the chemical reaction). The student’s firsthand experiences also serve to support ideas in the writing prompt perspectives.
Authoritative Explanations
Once we have an understanding on the conceptual level, it is time to develop a more sophisticated understanding of the chemical reaction that takes place on a microscopic level. First, we watch a video of how the molecules interact and recombine during the chemical reaction (Beaudry Interactive’s Video, 2015). I describe the chemical equation and challenge students to check the equation by making a T-chart to compare the number of atoms before and after the reaction (see Table 2). I had to explain what the subscript numbers revealed about the atoms involved in the chemical equation
NaHCO3 + CH3COOH → CO2 + H2O + NaC2H3O2
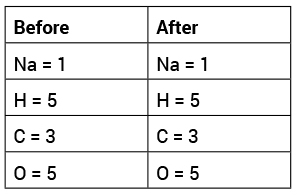
The T-chart helped students understand the disciplinary core idea (DCI) about conservation of mass and that the molecules and atoms involved in a chemical reaction can be used to predict the products of the reaction (NGSS Lead States, 2013; PS1.B: Chemical Reactions). In addition, the T-Chart helps make the lesson minds-on and students have to connect their observations to data. The connection between first-hand observations and teacher explanations helps promote the conceptual coherence of the lesson. Demonstrating that mass is conserved during a chemical reaction (Law of Conservation of Mass) provides the initial exposure and evidence to help students learn about chemical reactions.
Elaboration and Evaluation
I perform the Elaboration and Evaluation phase activities in tandem. I want students to engage in argumentative writing to confirm one perspective and refute another based on data. In this way, students can test their ideas in the context of the writing prompt. The scoring guide (Table 1) highlights key components of ACT-type questions and writing prompts, and students use their experiences to represent data (30–40% of the ACT) and write about conflicting viewpoints (15-20% of the ACT) (ACT, 2018). Our writing prompt goes beyond using science as a context; our goal is for the process to develop student content knowledge. I wanted students to demonstrate their content understanding by using skills similar to those asked for by ACT questions.
To promote their understanding (i.e., promote metacognition, and essential principal with how students learn best), I had students color code their argumentative essay using the highlighting feature to indicate when they were writing about conflicting viewpoints, presenting an accurate claim, providing evidence that supports their claim, and creating a reasoning statement (National Academies of Sciences, Engineering, and Medicine 2018).
The process of having students highlight their writing related to the scoring criteria is a beneficial way to help students focus and structure their writing related to the goals of the activity. A representative writing sample with highlighting is shown in Figure 2. Their writing emphasized the CCSS in ELA that suggests students “Write arguments to support claims in an analysis of substantive topics or texts, using valid reasoning and relevant and sufficient evidence (NGAC and CCSSO 2010; CCSS.ELA-LITERACY.W.9-10.1).”
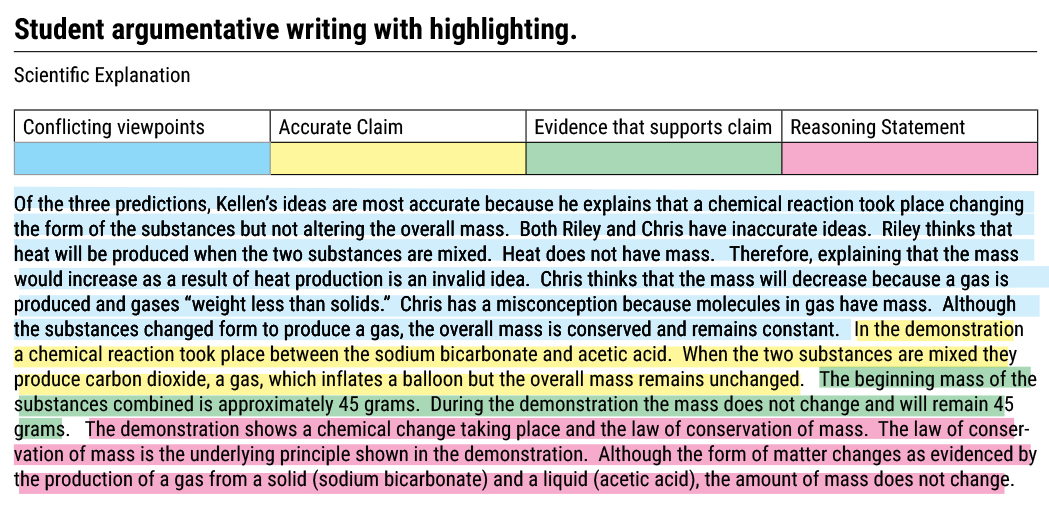
The final evaluation phase activity asks students to use the scoring guide to assess their writing. Following the student’s self-assessment, I scored their writing using the same criteria.
Conclusions
The combination of minds-on conceptual understanding, learning about chemical reactions, and creating a T-chart helps students bridge their macroscopic observation of phenomena with the macroscopic explanation. While teachers have been mixing baking soda and vinegar to show chemical reactions for some time, the explore-before-explain sequence and focus on argumentation writing is a way to maximize learning and highlight the three dimensions of the NGSS. In this way, the lesson highlights cognitive science research that shows that the learner- centeredness, assessment-centeredness, and knowledge-centeredness of a science classroom are important factors for the best possible learning environments (National Academies of Sciences, Engineering, and Medicine 2018).
Many school districts are pushing for change in the way science and all subjects are taught to better prepare students for college admission exams, such as the ACT. Interdisciplinary instructional approaches will be needed to assist students in developing proficiencies in test-taking strategies required of the ACT. While science is a component of the ACT, this section tests skills in close relation to the Science and Engineering Practices (SEPs) and their corresponding essential elements emphasized by the Next Generation Science Standards (NGSS) (NGSS Lead States 2013; Appendix F). The content used on the ACT creates a context for students to explore the following types and their representation on the test: Data representations (30–40%); Research Summaries (45–55%); and Conflicting View Points (15–20%) (ACT 2018). Also, there are optional ACT writing tests that assess students’ abilities to convey and develop ideas (ACT 2018).
Safety Notes
- Wear sanitized, indirectly vented chemical-splash safety goggles, nonlatex nitrile gloves, and a nonlatex apron during the set-up, hands-on, and take-down segments of the activity, for both students and teacher.
- Use caution when working with sharp objects (e.g., glassware). They can cut or puncture skin!
- Have direct adult supervision when working with hazardous chemicals.
- Immediately wipe up any liquid spilled on the floor—a slip-and-fall hazard.
- Wash your hands with soap and water after completing this activity.
Patrick Brown (plbtfc@gmail.com) is the Executive Director of STEM at Fort Zumwalt School District in O’Fallon, Missouri.
Chemistry Literacy NGSS Teaching Strategies High School


