feature
Is It A Change?
Assessments and demonstrations to challenge students’ conceptions about matter and encourage practice forming explanations
Science and engineering practices (SEPs) and crosscutting concepts (CCs) constitute a significant part of A Framework for K–12 Science Education (NRC 2012). Our role as teachers is to highlight the pivotal role that both scientific knowledge and the practices used to generate knowledge play in learning. This article shares how assessment probes can situate learning, drive firsthand experiences, and support deeper conceptual understanding.
This fifth-grade lesson targets two interrelated performance expectations (PE) that state students can, “measure and graph quantities to provide evidence that regardless of the type of change that occurs when heating, cooling, or mixing substances, the total weight of matter is conserved (5-PS1-2) and “conduct an investigation to determine whether the mixing of two or more substances results in new substances (5-PS1-4)” (NGSS Lead States 2013). The activities help confront student misconceptions about chemical reactions (Driver et al. 1994).
In grades 3–5, students can recognize when a substance changes forms; however, they have difficulty understanding that the parts of materials change their form during a chemical reaction or change. Although the chemical details are sophisticated, students can compare and contrast amounts of materials and their form before and after a chemical reaction. They can construct conceptual ideas about chemical changes creating new and different materials. This lesson is one step in helping students understand the conservation of matter that takes place during a chemical change.
This explore-before-explain lesson includes an assessment probe, demonstration, student’s evidence-based claims, and an enhancement activity from a technical reading. The use of explore-before-explain learning is a strategy that promotes conceptual change (Brown 2019). Conceptual change is a process where students develop their ideas and beliefs by using evidence to construct knowledge (Posner et al. 1982). The lesson took two 50-minute class periods and was used to engage students in learning about chemical changes and reactions. A modified version of this lesson will appear in the book Activating Students’ Ideas: Linking Formative Assessment Probes to Instructional Sequence (Brown and Keeley, Forthcoming).
The Assessment Probe
Students were engaged with an assessment probe that directly addresses misconceptions and lends itself to firsthand investigations (see Brown and Keeley, Forthcoming). In this way, the assessment probe situates learning about phenomena associated with specific content (disciplinary core ideas [DCIs]) while highlighting the role that asking questions, testing predictions, and carrying out investigations play in science learning (SEPs). The assessment probe was read out loud to the class (Figure 1).
Students made predictions about what they thought baking soda and vinegar would look like before and after they were combined. I showed students two beakers, one with 50-ml of vinegar and the other with 50-ml of baking soda. Students had various ideas of what the substances would look like and do when mixed. Many students thought the solution would “erupt” like a volcano (see Figure 2). Other students thought that the baking soda would dissolve in the vinegar.

The assessment probe: Is it a change?
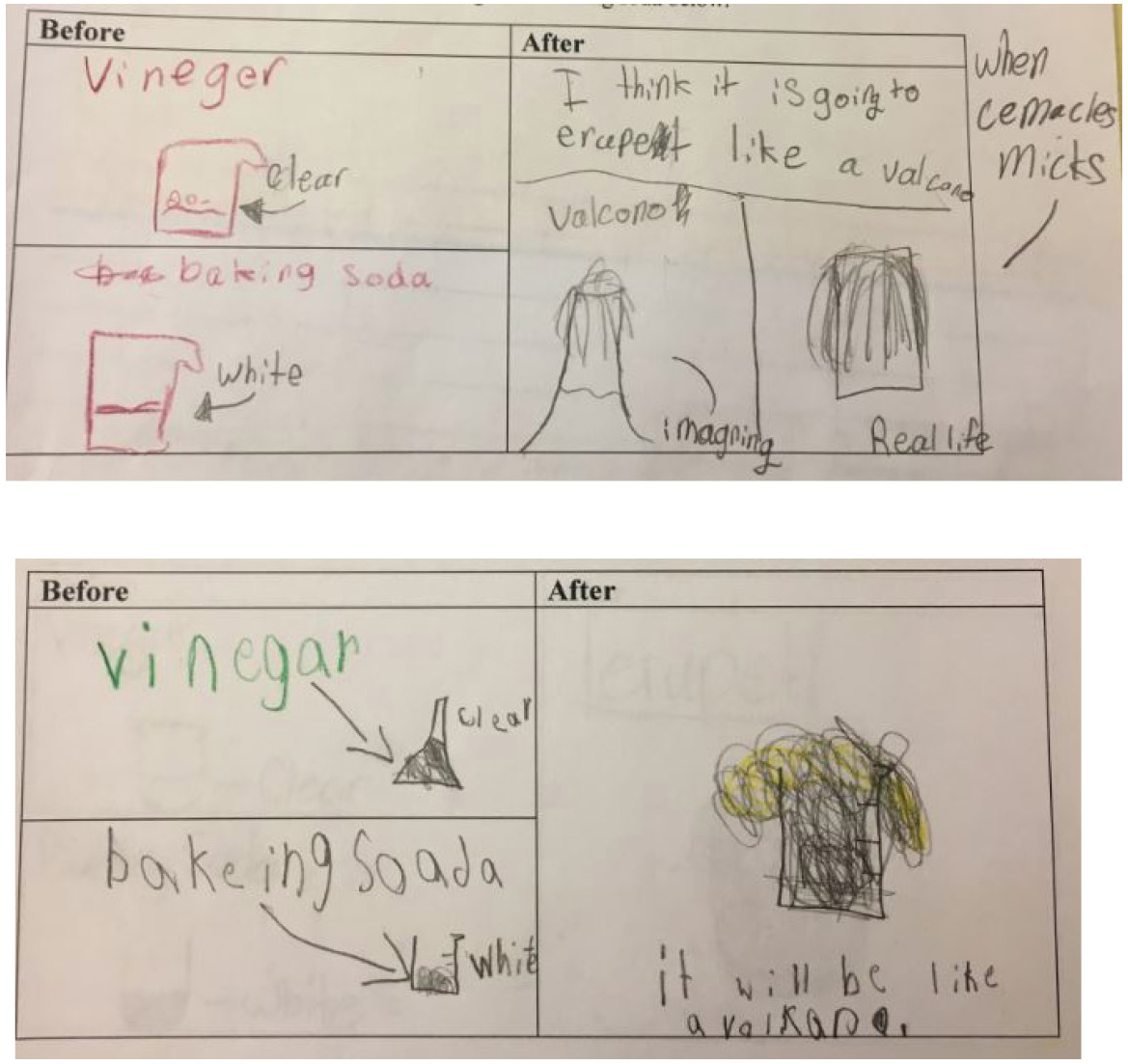
Student predictions.
I had two follow-up content questions for students that dealt with changes that may occur. The first dealt with changes in the masses of the materials and the second considered a change in temperature (see prior knowledge assessment probe in Figure 1, questions 1 and 2, respectively). Students had a range of ideas. Approximately half of the students thought that mass would be higher before the reaction. No students thought the mass would be the same.
Students’ ideas about change in temperature were also mixed. Fifty percent of students thought that the temperature would be higher after baking soda and vinegar were mixed. Twenty-five percent thought that the temperature would be higher before the two materials were mixed. Finally, 25% thought the temperature would be the same before and after the materials combined.
The last question asked students to give some thought to why they held certain conceptions. Most of the students’ responses were related to their past lived experiences (Items G, H, I, and K).
The formative assessment data lends fascinating insight for teachers. Many students said they had seen this demonstration (or something like it) before in a different class. Interestingly, while students may have seen the demonstration before, their mixed conceptions made me think that their prior knowledge did not provide them experiences that promote lasting understanding. Thus, this was a ripe area for investigations and my plan was for students to construct knowledge based on evidence they collect.
Hands-On Demonstrations
Once everyone had made a prediction, it was time to conduct a hands-on test. This “doing” of science requires a safe experience and indirectly vented chemical splash goggles are a requirement if students are performing the demonstration. Since I performed the demonstration, I wore goggles and had students sit approximately 2.5 meters from the front observation table. Many students found that the initial demonstration confirmed their predictions and the solution bubbled.
While not as dramatic as they had hoped for (no “eruption”), the demonstration helped us conduct more rigorous investigations of chemical reactions and focused on the cause-and-effect relationship evident in the chemical reaction.
We considered the questions, “When we mixed baking soda and vinegar into a single solution, did we create a new and different substance?” and “What evidence do we have to support our ideas?” We talked about the before and after substances in terms of states of matter. Students explained that we mixed a solid (baking soda) to create a liquid and a gas. The bubbling and production of gas was an excellent way to target the CC of cause and effect and provided some beginning evidence that the solution had new and different properties (one being the production of gas). At this point we questioned whether a gas has mass and how we could measure it in an investigation. While the initial demonstration was anticlimactic, the conversation that followed intrigued students about the changes that occur.
To delve into the variables from our formative assessment probe (mass and temperature), we needed to conduct further testing. To investigate changes in mass, I put 50ml of vinegar in an Erlenmeyer flask. Next, I put baking soda in a balloon. I carefully put the balloon on the Erlenmeyer flask, making sure not to mix the baking soda and vinegar. I used some black electrical tape to seal the balloon to the Erlenmeyer flask and make sure there were little to no gaps. Finally, I placed the flask-balloon set-up on an electronic balance (see Online Resources for the investigative setup). When the teacher lifts the balloon and gently shakes the baking soda out of the balloon and into the vinegar, they initiate the chemical reaction. (Note to teachers: This demonstration is challenging to set up and mimics a closed system. Teachers should perform the demonstration beforehand because if the system is not as closed as possible, gas will be released, promoting misconceptions about conservation of mass). We performed the demonstration on the electronic balance. (Teachers should wear protective goggles, a heavy-duty apron, and gloves.) To students’ surprise, while the balloon inflated, the mass did not change (mass remained 2.940 grams).
The data highlighted the importance of the decimal places in this measurement and each of the numbers—before and after the decimal—were important evidence needed to help us formulate our claim. The focus on the crosscutting concept of scale, proportion, and quantity was a nice bridge to the CCSS-Math standard that emphasizes students gain abilities to represent a “data set of measurements in fractions of a unit” (NGAC and CCSSO 2010; CCSS.MATH.CONTENT.5.MD.B.2).
The next demonstration was designed to test whether a change in temperature occurs during a chemical reaction. Equal amounts of baking soda and vinegar were placed in beakers. While typically you would use a volume to measure a liquid, having equivalent masses was an easy way to illustrate that we were using the same amounts of substances. Any equivalent amounts would work for this demonstration). Next, the temperature was taken of each solution. The baking soda was 22.5°C and the vinegar was 21.2°C. The two materials had slightly different temperatures. When the materials were mixed, to student’s surprise, the temperature decreased significantly, to 14.5°C.
Evidence-Based Claims
At this point in the lesson, I wanted students to articulate their understanding through writing. I wanted to push students to think about why conservation of mass occurs during chemical reactions in a closed system. As a class, we all had the same evidence related to the phenomenon. I asked students to make a Claims-Evidence-Reasoning (C-E-R) statement to encourage students to construct explanations for phenomena. The first part of students’ C-E-R statement was aimed at explaining just the claim they could construct based on evidence they witnessed firsthand. In this regard, students’ claims typically represent what they can explain on a conceptual level about science. One student wrote, “The weight stayed the same, but the temperature went down.” At this point, students’ evidence-based claims were easily assessed because they were either correct or incorrect based on the data observed.
I also challenged students to write a reasoning statement for why what they observed happened. To help guide students with the reasoning statement, I used a sentence frame so they would know how to participate in technical writing. I wrote on the board, “I think the weight staying the same has something to do with _______” and “the temperature decreased when the two substances are mixed had something to do with ________.” The sentence frames were a way to scaffold this difficult task and accommodate students with limited English abilities. Students had very similar ideas for both of their reasoning statements. One student wrote that they thought the mass remained the same because “the materials were all still there but just in a different form.” For the second sentence frame, a student explained, “the temperature decreased because the materials went from solids and liquids to some gas.” I was pleased with students’ reasoning statements at this point. Their thinking was important for developing future understanding and would be assessed after more elaborate explanations.
Enhancements
The enhancement activity allowed students to build on their prior experiences and knowledge by providing accurate content from a reliable source. The authoritative explanation included a short reading and teacher-directed explanation. We read a short excerpt from a grade 3–4 reader titled, The Scoop about Measuring Matter:
“Mass stays the same when an object changes physical properties, such as state, color, or shape. Mass also stays the same in a chemical reaction, when two combined materials change into entirely new material” (Duke 2013).
The Law of Conservation of Mass
In 1789, French chemist Antoine Lavoisier proved that mass put into a reaction equaled the mass that came out of the reaction. No new mass. No mass destroyed. Voila, you have the conservation of mass (Duke 2013).
Thus, students formed a more elaborate understanding that the mass is conserved in a chemical reaction and this property is termed The Law of Conservation of Mass.
The teacher portion of the explanation dealt less with why the temperature decreased (the endothermic change goes beyond grade 3–5 content and is appropriate for high school) and focused on signs of chemical changes. In addition, the short reading verified students’ firsthand experiences with an explanation from a credible source. For the class, I listed common indicators of chemical changes that included: formation of gas bubbles, color change, and temperature change.
Thus, students inferred from the demonstration and temperature data that a new substance was produced that has different properties than baking soda and vinegar. Once students had a deeper understanding, I had them add to their reasoning statements. My goal was to wed students’ firsthand experiences with data and authoritative explanations so student construction of knowledge was elaborated to include more scientific understanding. Students were able to add to their reasoning statements. One student wrote, “because temperature change is a sign of a chemical change, I know a new substance was made in the demo.” I also had students go back to the formative prior knowledge assessment probe and reflect on their developing understanding. I asked students to complete the sentence frame: “At first I thought _________ and now I think _________ because ________. Students’ C-E-R statements were my summative evaluation of their learning and a way to bridge science with the Common Core State Standards (CCSS) in English Language Arts (ELA) that suggest that students “provide a concluding statement or section that follows from and supports the information or explanation presented” (NGAC and CCSSO 2010; CCSS.ELA-LITERACY.W.5.1.D). In my evaluation, I assessed whether students had an accurate evidence-based claim and used information from the lecture as a reasoning statement that helped describe the underlying properties and signs of chemical changes.
Conclusion
In a crowded school day, where every instructional minute matters, explore-before-explain teaching can maximize learning. The connection between activities in the Is It a Change? lesson highlights the pivotal role that instructional sequence plays for learners. The sequence of assessing knowledge using tools that situate learning and directly lend themselves to explorations is a way to make science relevant. Next, simple shifts in the arrangement of hands-on activities to the beginning of new units of study can make a big difference for learners. By allowing students to first construct conceptual knowledge through firsthand experiences and articulate their understanding through evidence-based claims before developing ideas through explanations is paramount to an explore-before-explain approach (Brown 2019). In this way, new knowledge is deeply entrenched in their firsthand experiences.
While the sequence of explore-before-explain is not new, the sequence may take some time to fully master. The most critical part is deciding on the knowledge students can construct from their experiences and the ideas teachers need to introduce to create more elaborate understanding. Once teachers have pinpointed some aspect of knowledge students can construct, they can create formative assessments that address students’ prior experiences and knowledge and lend them to firsthand investigations of phenomena. When teachers use students’ experiences with data as the hook for developing more sophisticated understanding, they are collaboratively building understanding in ways aligned with how students best learn science (National Academies of Science, Engineering, and Medicine 2018).
Acknowledgment
I thank Page Keeley for her collaboration on this lesson.
Patrick Brown (plbtfc@gmail.com) is executive director of STEAM and CTE with the Fort Zumwalt School District in O’Fallon Missouri.
Crosscutting Concepts Disciplinary Core Ideas NGSS Performance Expectations Science and Engineering Practices Three-Dimensional Learning Elementary



