science 101
Q: How Does the Sun’s Heat Get To Us Here on Earth?
A: It doesn’t. Does that answer surprise you? It might surprise your students, too, but I’ll explain. First, remember what heat is. Heat is the flow of energy from one object to another object because of their difference in temperature. And that energy—thermal energy—results from the random motion of atoms and molecules. The molecules move around, bouncing off of each other and bouncing off anything with which the object comes into contact. The hotter the object, the faster the molecules are moving.
If you have a hot pot on the stove, the molecules in that pot are moving around very fast. If you touch that pot with your finger, some of those fast-moving molecules in the pot slam into the molecules in your skin! Just as a fast-moving bowling ball transfers lots of energy to the pins, the pot molecules transfer lots of energy to your skin molecules, making them move very fast. And, having fast-moving molecules in your skin means that your skin is now hot. At a microscopic level, that’s why you get burned when you touch a hot pot (see Figure 1, and don’t try this at home).
Don’t touch a hot pot lest the fast-moving pot molecules smack into your finger’s molecules.
Now, we all know that the Sun is hot. How hot? Well, the layer of the Sun that you would see if you glanced up at it—which I do not recommend—known as the photosphere (also, misleadingly referred to as the “surface of the Sun,” as there is no solid surface) has a temperature of around 6,000°C (11,000°F). So, the atoms there are moving exceedingly fast. But, fortunately for us, those super-speedy atoms do not come into contact with us, and their kinetic energy doesn’t get transferred to us. That’s why I started my answer to the original question with, “It doesn’t.” It’s not thermal energy—energy from the movement of the atoms—that reaches us here on Earth.
Energy From the Sun
What does get to us from the Sun, providing the vast majority of the energy we use, is energy in the form of light, or, more generally, electromagnetic radiation, which includes:
- Radio waves
- Microwaves
- Infrared radiation
- Visible light
- Ultraviolet radiation
- X rays
- Gamma rays
These are all kinds of light but with different wavelengths, and most of these are not kinds of light that our eyes can detect. We see only a small part of all of this radiation—only the visible light.
Most of the energy that we receive from the Sun arrives here in the form of infrared, visible, and ultraviolet light, although the Sun does emit small amounts of all the other kinds of radiation as well. The sunlight that reaches Earth’s surface is around 53% infrared, 43% visible light, and 4 percent ultraviolet radiation (see Figure 2).
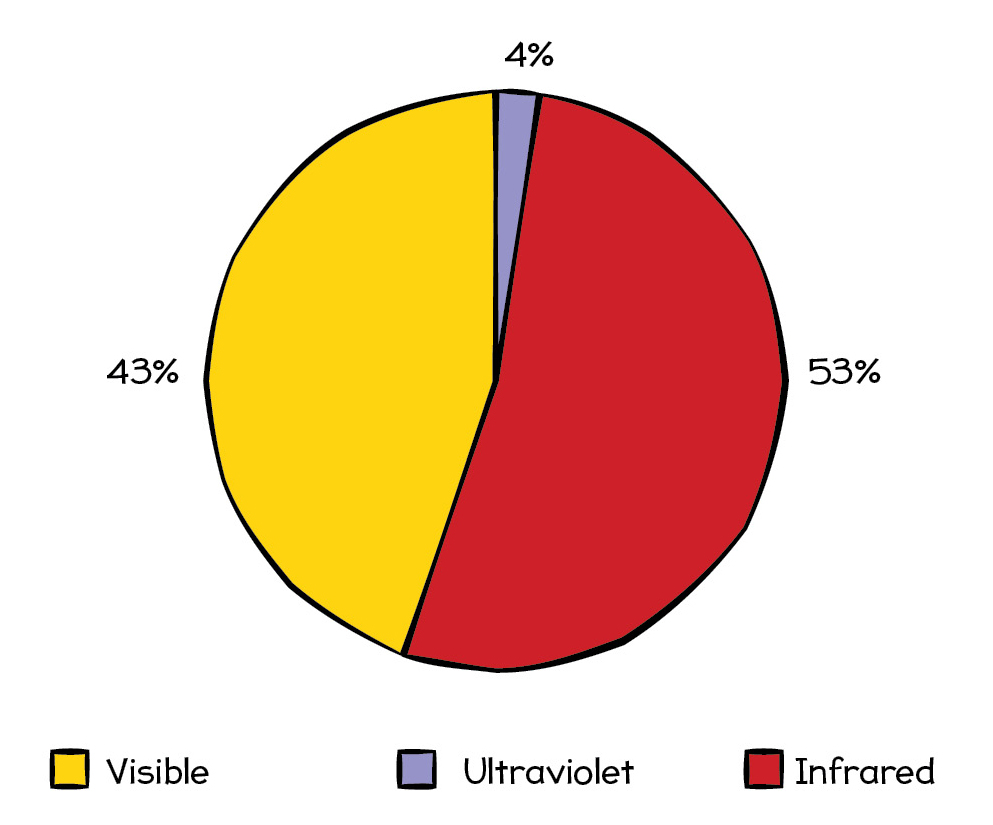
The sunlight that reaches Earth is split almost evenly between visible light and infrared, with only a few percent being ultraviolet.
When all of that energy reaches the Earth, some of it gets absorbed and some of it gets reflected. The reflection of some colors of visible light more than other colors causes objects to appear to have different colors. We see the colors that are reflected. But it’s the light (or radiation) that gets absorbed that is responsible for heating the Earth—or you. When you stand out in the sunlight and feel the warmth on your skin, you are feeling thermal (or heat) energy, of which about half came from your skin absorbing infrared radiation, a bit less than half from your skin absorbing visible light, and a few percent from your skin absorbing ultraviolet radiation. The light that is absorbed disappears, and the energy from that light becomes thermal (or heat) energy.
We saw in a previous Science 101 article (March/April 2021; see Online Resources) how materials of different colors will absorb different amounts of light and therefore produce different amounts of heating. Next, let’s try an investigation to see how the absorption of radiation from the Sun can heat a house.
An Investigation: Solar-Heated and Insulated Houses
Divide the students into groups (at least two), and have each group make a house out of cardboard and tape. Except, half of the groups will make houses with many windows or large windows, and the other half will make houses with no windows. The houses with windows should have the windows covered with plastic wrap, preferably taped on the inside (for aesthetic reasons). Houses can be decorated as an additional art project (or to make it a STEAM project; see Figure 3). You or another adult might need to assist with the cutting of the cardboard. The houses with no windows can be further insulated with materials such as aluminum foil or batting.
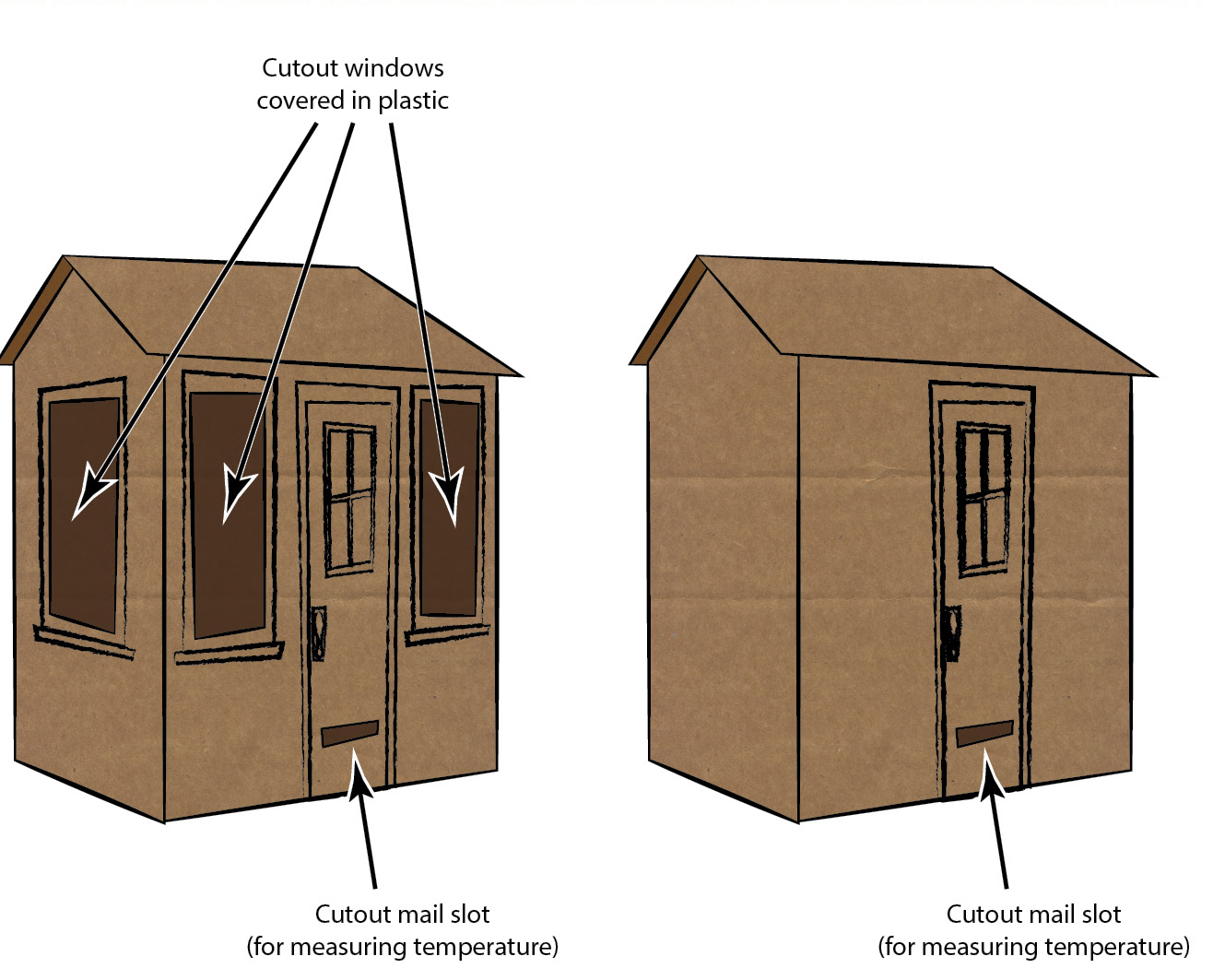
Cardboard houses (a) with, and (b) without windows.
You’ll also need some thermometers, which will be used to measure the temperature in each house and to measure the temperature of the outside air. If different houses have different kinds of insulation, the thermometer will reveal what types of insulation work best for keeping the house warm or cool. A hole can be poked through the cardboard (or through the mail slot in the door) to allow a thermometer to stick into the house.
If your classroom has windows, students can place the cardboard houses in the direct sunlight coming in the windows. It will take a while (check every half hour) to notice much of a temperature change, and you’ll have to periodically move the houses to keep them in the sunlight. If you don’t have windows, then you can carry out this investigation outdoors when the weather is suitable.
Students should record their data. Afterward, have them discuss what they’ve learned—about what makes good insulation and about how solar energy—sunlight—gets absorbed and converted to thermal (heat) energy.
One point that students can discuss is how insulation is helpful in both summer and winter. In summer, insulation helps keep the heat out, so the inside of the house doesn’t get too hot (and you won’t need to spend as much on air conditioning, if you have it), while in winter insulation helps keep the heat in so that the house doesn’t get too cold (and you won’t need to spend as much on heating).
Sunlight is most intense when the Sun is in the south (because that’s when the Sun is highest in the sky), so if you live in a cold climate and want sunlight to help warm your house, you’ll want to have some large windows facing south. Don’t have them shaded during the cold weather when you can use the solar heating, but shade them in the summer to avoid overheating.
What materials are in your home can help as well. Certain materials, such as concrete, brick, stone, and tile, absorb heat from sunlight during cool weather—the heating season—and these materials absorb heat from warm air in the house during hot weather—the cooling season.
Have you discussed conduction, convection, and radiation? Thermal (heat) conduction occurs when heat moves between objects that are touching each other, such as when a sunlight-heated floor warms your bare feet. Fans can help move air (convection) from warmer areas—a sunroom, for example—into the rest of the house. Radiation is what you feel when you stand next to a sunny window and feel its warmth on your skin. And darker colors absorb more heat than lighter colors.
If you haven’t yet experienced the benefits of absorbing solar radiation, I think you’ll warm up to the idea.
Never stop learning.
Online Resources
Science 101: What is Energy?
Science 101 article with experiment on converting light into heat for different colors
Matt Bobrowsky is the lead author of the NSTA Press book series, Phenomenon-Based Learning: Using Physical Science Gadgets & Gizmos. You can let him know if there’s a science concept that you would like to hear more about. Contact him at: DrMatt@msb-science.com.
Phenomena Physical Science Elementary



