Science 101
Q: What’s Cool About Water?
A: One answer could be ice, for several reasons. Show students an ice cube and ask them what its temperature is. Younger students might have no idea. Older students might say 0°C or 32°F, because they’ve heard that’s the temperature at which water freezes. In fact, if you just took the ice out of a freezer, then the temperature of the ice is whatever the temperature of the freezer is. The ice in my freezer has a temperature of –10°C (14°F), because that’s the temperature inside my freezer. Cool, huh?
Water is one of the few substances that expands when it freezes. Since it expands when it freezes, its density becomes lower, which is why ice cubes float on water. That’s also why ice forms on the surface of a lake or river, with liquid water underneath the ice. If ice didn’t float, then smaller lakes and rivers could freeze solid in the winter, killing all life in the water. So, it’s good for aquatic life that water expands when it freezes into ice, which floats.
A water molecule consists of two hydrogen atoms and one oxygen atom, so we write its chemical formula as H2O. This is actually one of the most common molecules in the universe, and liquid water is essential for life on Earth.
Water as a Solid, Liquid, or Gas
Water is a good example to use when discussing how matter can be in different states—solid, liquid, or gas. Students are quite familiar with solid water (ice) and liquid water. Gaseous water (or water vapor) is a bit more abstract, because you can’t directly see it. It’s only after the vapor condenses into liquid water droplets, that you can see the water as fog or mist. A cloud is an example of this, as it’s made up of countless tiny (liquid) water drops.
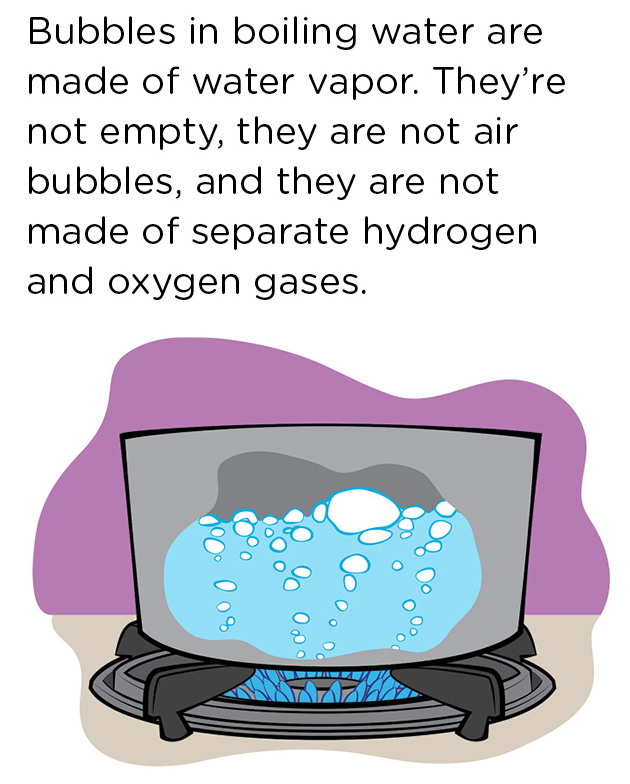
If your students have seen a pot of boiling water, ask them what they see in the water. The answer should be “bubbles.” The next question is, what are the bubbles made of, or, what’s inside the bubbles? Incorrect answers would be “nothing” or “air.” It’s understandable that young students might guess there’s air in those bubbles since when they blow bubbles with a soap or other bubble solution, they’re actually blowing air into the bubbles. But, with the pot of boiling water, nobody is putting air into the water. So what’s in the bubbles? The hot burner under the pot heated the water to the boiling point, which is the temperature at which liquid water becomes water vapor. So those bubbles in the pot of water are made of water vapor, i.e., water in the form of a gas (Figure 1).
Surface Tension
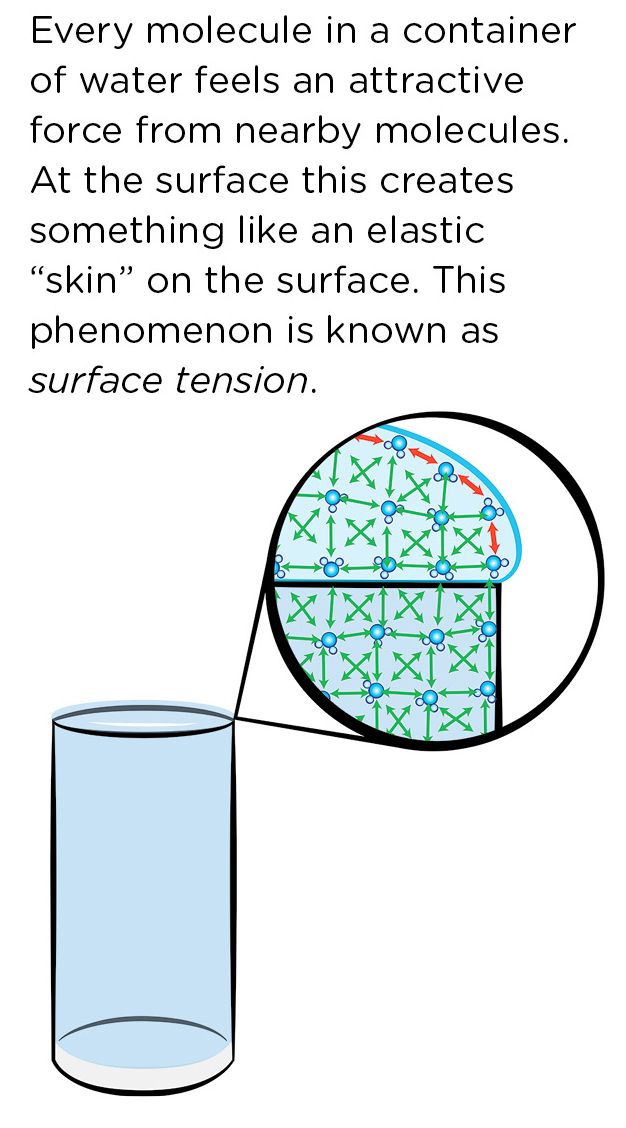
In the October 2018 Science 101 column, I discussed electric charges and how opposite charges attract each other, and like charges repel each other. Water molecules have a weak attraction to one another, as shown by the arrows in Figure 2. The red arrows indicate the forces along the surface, which makes the surface behave a bit like an elastic “skin.” This skin allows the glass of water to be filled above the brim without the water spilling over.
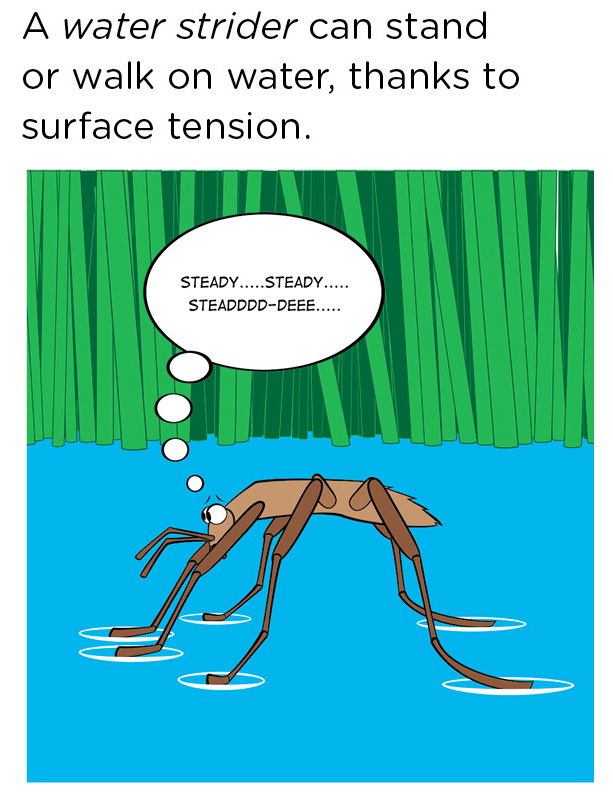
The elastic nature of the surface of the water allows an insect called a water strider to actually walk on the surface of the water (Figure 3).
Surface Tension Investigations
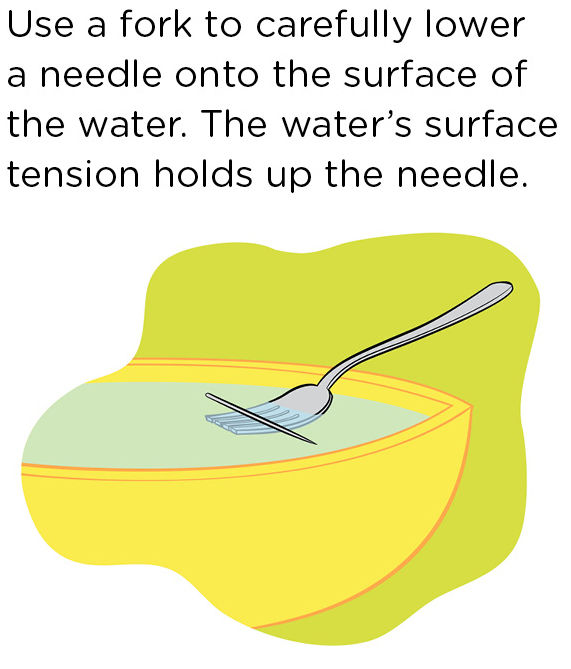
You and your students can demonstrate surface tension for yourselves. You know that metal, e.g., steel, is dense and normally will sink in water (unless you make a boat out of it). So students might be surprised to see that they can make a piece of steel appear to float on water. They can do this with a small piece of metal, such as a sewing needle or a paper clip. It’s probably easiest with a small needle, in which case eye protection should be used. Start with a bowl of water filled to the brim. Carefully place the needle on a fork (Figure 4), and slowly lower the fork into the water. If you do this carefully, you can have the needle held up by the water’s surface tension. Carefully slide the fork out from under the needle and out of the water. Don’t make any waves!
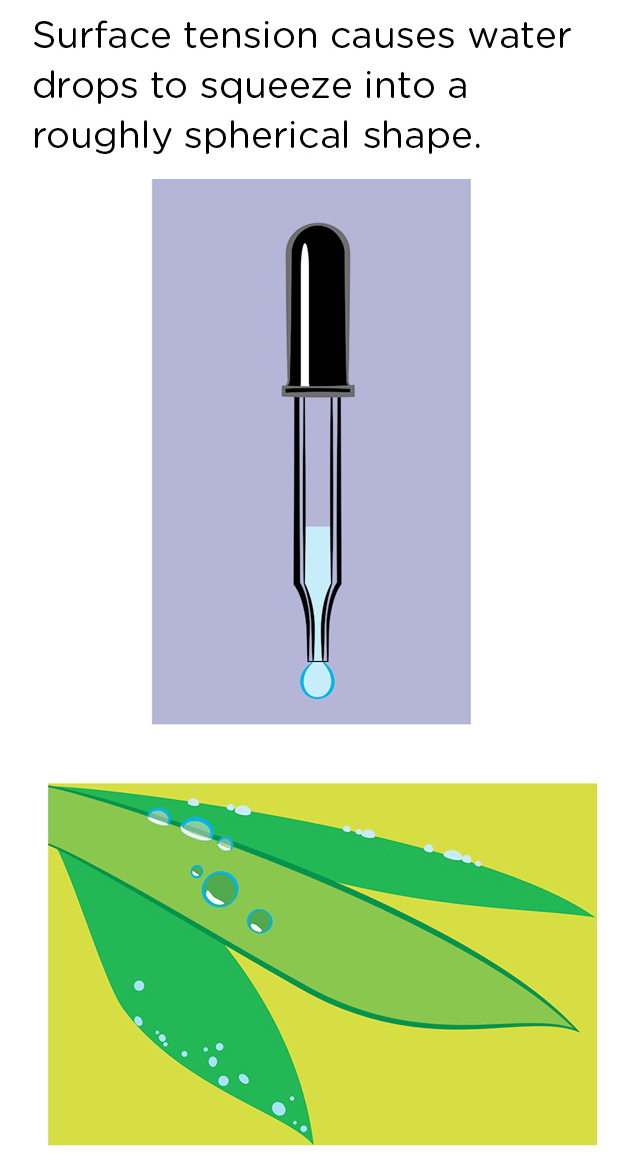
The forces of surface tension tend to pull drops of water into a spherical shape, because a sphere is the shape that is most compact, i.e., has the smallest surface area possible. You can see this (roughly) spherical shape if you start to squeeze a little water out of a dropper or if you look at drops of water on a leaf (Figure 5).
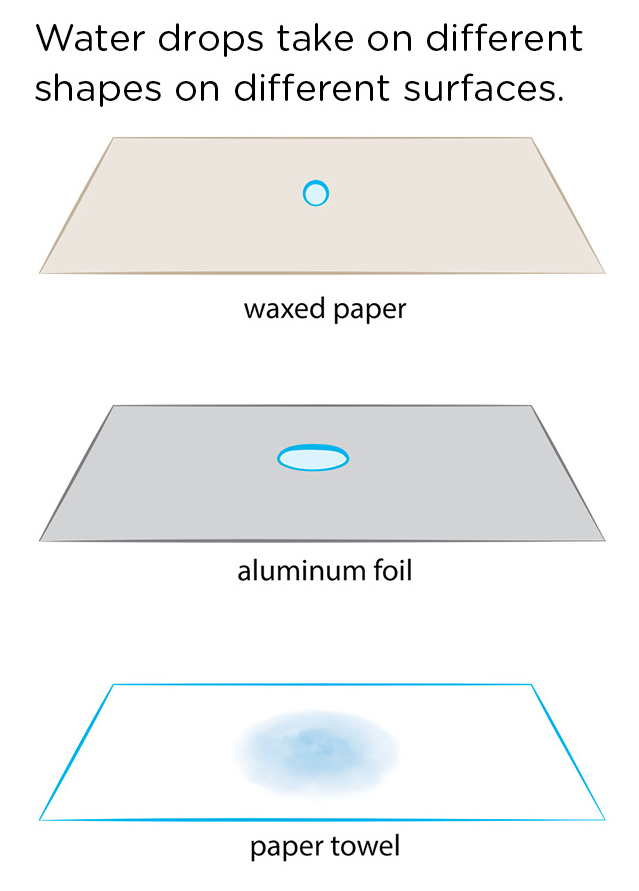
On some surfaces, the water droplets will not ball up and will spread out instead. These are surfaces on which the water’s attraction to the surface (adhesion) is greater than the water molecules’ attraction to each other (cohesion). A quick but interesting investigation involves having students put drops of water on different surfaces to see where the water drops form round balls and where the water drops spread out. Surfaces to try include wax paper or parchment paper (drops ball up), aluminum foil (drops spread out), and paper towels on which the drops spread out quite far due to capillary action (as discussed in the chromatography investigation in the July 2020 Science 101 column; see Figure 6). Students can put drops of water on other surfaces as well, and after a while, might get good at predicting what the water drops will do on various surfaces. What would you expect a drop of water to look like on a raincoat? More like the waxed paper or more like the paper towel? Why are raincoats made out of a material like that?
As raindrops say, two’s company, three’s a cloud.
Never stop learning.
Online Resources
Bobrowsky, M. 2018. Science 101: What Is “Static Electricity,” and How Can I See Its Effects? Science and Children 56 (3): 64–67.
https://www.nsta.org/q-what-static-electricity-and-how-can-i-see-its-effects
Bobrowsky, M. 2020. Science 101: What’s a Fun Activity That Combines Science With Art? Science and Children 57 (9): 70–73.
https://www.nsta.org/science-and-children/science-and-children-julyaugust-2020/whats-fun-activity-combines-science-art
Matt Bobrowsky is the lead author of the NSTA Press book series, Phenomenon-Based Learning: Using Physical Science Gadgets & Gizmos. You can let him know if there’s a science concept that you would like to hear more about. Contact him at: DrMatt@msb-science.com
Biology Earth & Space Science Instructional Materials