feature
The Molecules That Make Me Unique
CONTENT AREA Physical Science
GRADE LEVEL 6–8
BIG IDEA/UNIT Atoms, molecules and compounds
ESSENTIAL PRE-EXISTING KNOWLEDGE None required
TIME REQUIRED 95 minutes
COST Minimal (<$20)
SAFETY Students should wear safety goggles when working with models.
As a fifth-year teacher in an urban middle school, I have become keenly aware of how important it is to provide my students, who are predominantly African American, with culturally responsive classroom experiences. Unfortunately, there are not many resources that support the application of these approaches to science instruction.
Then one summer I was selected to participate in a Research Experiences for Teachers (RET) program. The program gave me an opportunity to conduct lab-based research. It also required that I (along with nine other teachers) develop a culturally responsive curricular module, applying what I learned to one of my science courses. But I still had some concerns. What would it look like when implemented? Would there be time to include a cultural focus in addition to the science content? Would the cultural focus distract from the science content? How would students respond? In this article, we describe a two-part lesson in which I employed socially transformative science curriculum as one means of being culturally responsive. I also conclude with some of the benefits realized from the experience.
What is socially transformative science curriculum?
The goal of socially transformative curriculum is to help children develop the tools they need to “transform” the society in which they live (Freire 1970; Pitts Bannister et al. 2017). I especially like this goal for the students that I teach because many hope to one day change their social circumstances. Many opportunities exist in the traditional science curriculum for teachers to help prepare science learners for the long-term goal of social change.
To provide some guidance, there are five areas of mastery that we want to help students to achieve (Mutegi 2011). First, students should develop a mastery of content. The bulk of our instructional focus is aimed at helping students to understand content, and content mastery is essential for students to have access to science and science-related professions. Second, students should develop a mastery of currency, which is to say that they should develop an understanding of how the content is relevant to human beings. Third, students should develop a mastery of context, which refers to students’ understanding of how the content is related to them. This area of mastery is important because it helps students to see themselves represented in the curriculum. This can be accomplished by introducing them to significant people, places, or events (whether historical or current).
Fourth, students should develop a mastery of critique, which refers to students’ ability to be critical consumers and producers of scientific knowledge. Here students should learn to recognize shortcomings, unseemly influences, and flaws in scientific practice and in the application of scientific knowledge. Finally, students should develop a mastery of conduct, in which they learn practical skills for the application of science knowledge. It is not necessary for each lesson to encompass all five areas of mastery. The goal should be to infuse as many as are reasonable.
In this application of socially transformative science curriculum, I began with a traditional lesson on atoms, molecules, and compounds and modified two activities within the lesson. The first modification was aimed at helping students develop mastery of context, which in this case refers to an understanding of how atoms, molecules, and compounds relate to them as people of African descent. The second modification was aimed at helping students develop a mastery of critique, which encouraged them to pay close attention to the limitations of the models we use to represent atoms, molecules, and compounds. Both modifications are consistent with Mutegi’s (2011) description of socially transformative STEM curriculum, as well as the Next Generation Science Standards’ (NGSS Lead States 2013) emphasis on foregrounding culture (National Research Council 2012, pp. 306–307) and critiquing (National Research Council 2012, p. 77) and identifying limitations of models (National Research Council 2012, pp. 56–58).
Engagement (10 minutes)
This is the first lesson in the chemistry unit. Typically, students are familiar with the Bohr model of the atom. Students also come with the idea that atoms are small and that they make up matter. By contrast, they do not typically have an idea of the various visual models used to represent molecules. They are largely unfamiliar with ball-and-stick models for molecules and compounds. For many, it is their first opportunity to create and manipulate these models.
To begin the engagement, I project an image of the three-dimensional model for a melanin molecule on the screen. This image is also provided on the first page of the students’ journal entry with the caption, “What do you think this is?” Students are instructed to write a description of what they see in their journals. An example of a student’s journal entry is shown in Figure 1. The image I use for the melanin molecule is taken from PubChem (see Resources). Once students complete the first part of the journal entry, I reveal that they are looking at the compound melanin. I then use open-ended questions to engage the class in a discussion of the biological role of melanin: Has anyone heard of melanin? What does melanin do? Why is melanin important? What are the properties of melanin? During the discussion, students take notes on melanin in their journals.
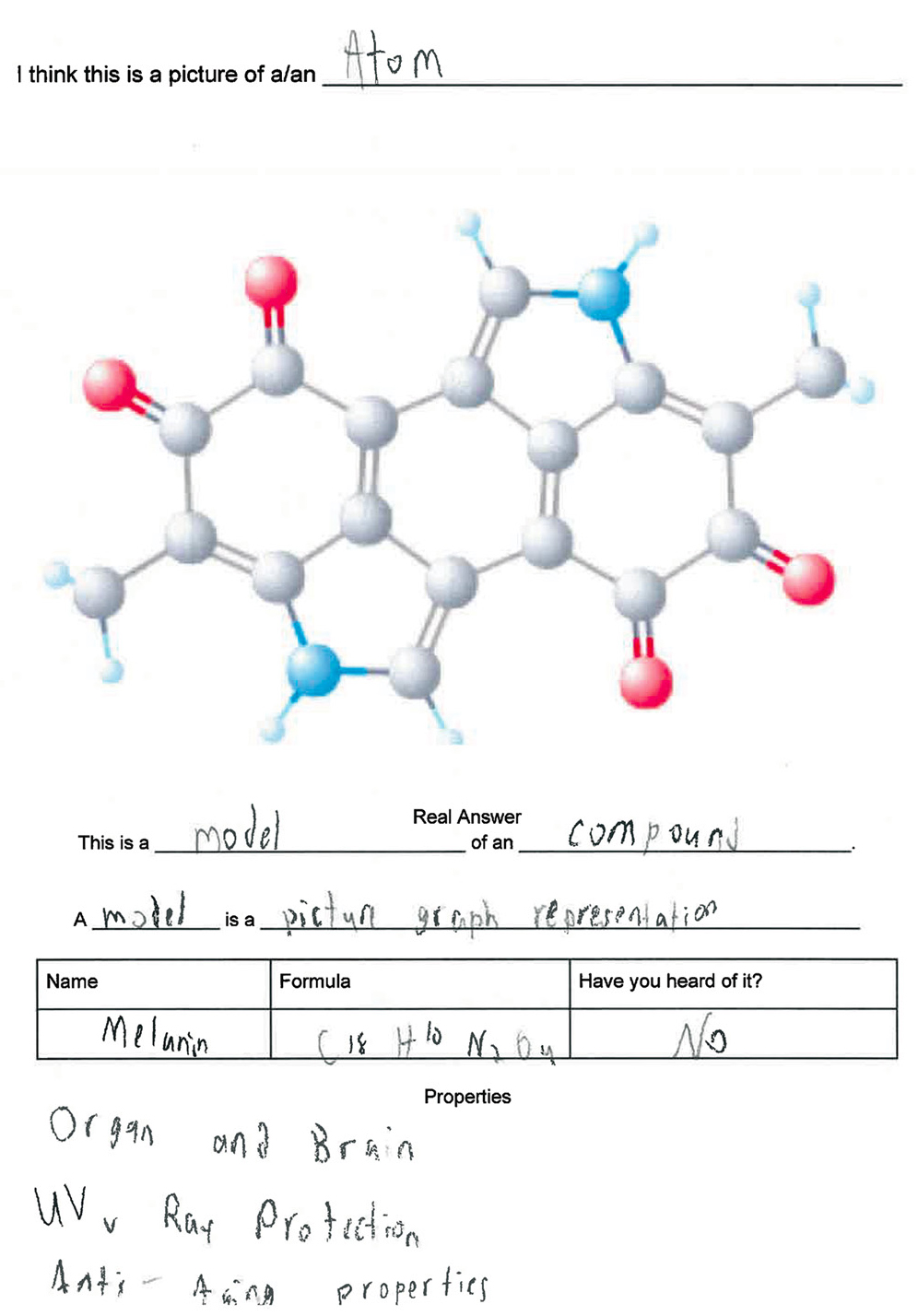
Student journal entry for engagement exercise.
In guiding the discussion, there a few points I underscore. On the biological role of melanin, I make sure to emphasize that (a) melanin is a pigment found in nature; (b) in humans melanin is reflected in skin, hair, and eye color; (c) nonhuman mammals and some plants also have melanin; and (d) greater amounts of melanin are responsible for darker colors. On the chemical classification of melanin, I make sure to emphasize that (a) melanin is a compound; (b) compounds are made of two or more different elements; and (c) the elements seen in our melanin compound are carbon, hydrogen, nitrogen, and oxygen. On the importance or benefits of melanin, I make sure to emphasize that (a) melanin is essential to brain, nerve, and organ function; (b) it provides protection against ultraviolet radiation; and (c) it promotes younger looking skin in humans.
This engagement could be conducted with any number of compounds. I considered both glucose (the simplest monosaccharide and a key component of many carbohydrates) and capsaicin (one of the molecules that make peppers hot). However, I use melanin because it provides an opportunity to help students develop an understanding of how the content relates to them as people of African descent (context).
Exploration (30 minutes)
For the exploration portion of the lesson, I provide students with red, yellow, and blue modeling clay; toothpicks; trays; and a list of five chemical formulas (C, H2O, H2, CO, and CO2). Working in groups of three, their first task is to form the clay into balls, each ball representing an atom. Each color represents either carbon, hydrogen, or oxygen. Using the clay and toothpicks, students create models reflecting each of the formulas provided. They also indicate whether each is an atom, molecule, or compound. After creating their models, students collaborate with their groupmates to create graphic organizers representing the five chemical formulas. Each group is expected to explain to the class the rationale behind the organization used.
Explanation (20 minutes)
The explanation portion of the lesson is aimed at facilitating students’ understandings of the differences between atoms, molecules, and compounds. To begin this portion of the lesson, students are provided with a Venn diagram featuring bubbles for molecules, compounds, and atoms as well as brief descriptions of each (see Figure 2). A version of this diagram is projected for the whole class to see. I then lead the class in a discussion during which we write the chemical formulas on the diagram to indicate where each of the five models should be placed. After adding our five formulas to the diagram, I provide students with six new formulas (NaCl, Ag, Co, SO2, H2, and C18H10N2O2). Working in their groups, students decide where these formulae should be placed on the Venn diagram.
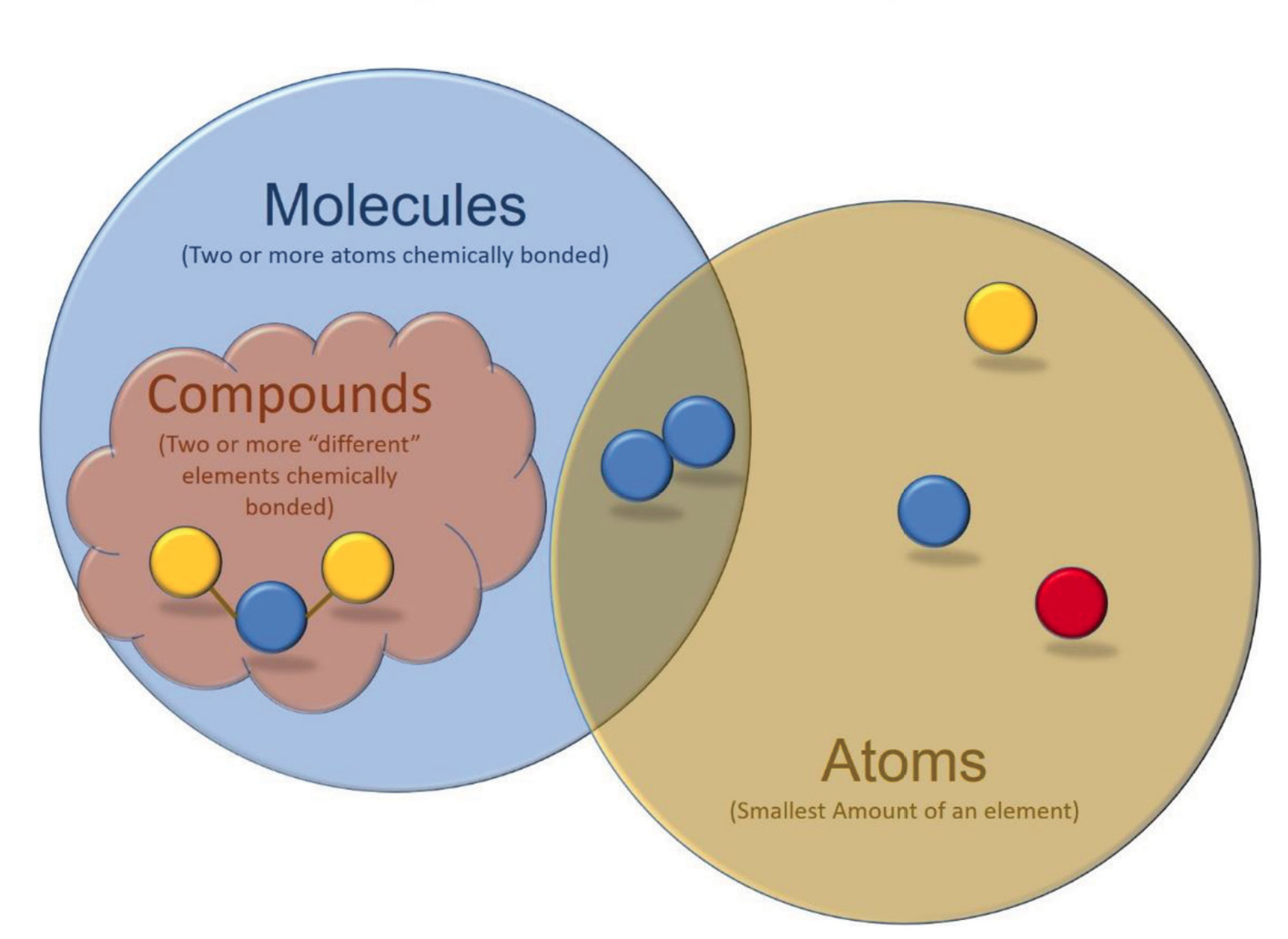
Venn diagram for molecules, compounds, and atoms.
Elaboration (20 minutes)
At this point in the class, I have provided students with 11 chemical formulas. For the elaboration portion of the lesson, I ask students to identify the common or scientific names for as many of these formulae as possible. I then ask students to record answers to two questions in their journals. The first question asks, “How are the clay models similar to (and different from) actual atoms, molecules or compounds?” The second question asks, “What are the advantages and disadvantages of using clay models to represent atoms, molecules, or compounds?” This portion of the lesson concludes with a discussion of the strengths and weaknesses of scientific models. Typically, the types of disadvantages students pinpoint are inaccuracies in the scale of our models (e.g., “in our models the distance between atoms is fixed” or “our models do not represent the relative sizes of atoms”) and the incompleteness of our models (e.g., “our models do not show different types of bonds” or “our models do not show components of atoms”). I draw from their comments to help them see that they are identifying inaccuracies in scale or incompleteness in models. These larger themes are revisited throughout the course when examining other scientific models.
These questions and the related discussion provide a good opportunity to support students’ efforts to become more critical consumers and producers of scientific knowledge (critique). Because students are readily able to see limitations in their own representations (models), I use this as a starting point for helping them to see limitations in scientific representations (models). The models that scientists use to represent the world are always incomplete. They are tentative. They are not fully accurate. They leave a lot unanswered. While these characteristics do not invalidate scientific models, they do encourage us to be measured when we think about how fully a model represents the natural world.
Evaluation (15 minutes)
Although students participate in a number of formative assessments throughout this lesson (e.g., small- and whole-class discussion), the journal entry and the Daily Science Review (see Online Supplemental Resources) are the culminating evaluation activities. For their final journal entries, students first complete a table wherein they identify a substance, its chemical formula, its classification (i.e., element, molecule, or compound), and a two-dimensional model of the classification. Following that, they summarize their understanding of the limitations of models. Figure 3 provides a student’s example of this final journal entry.
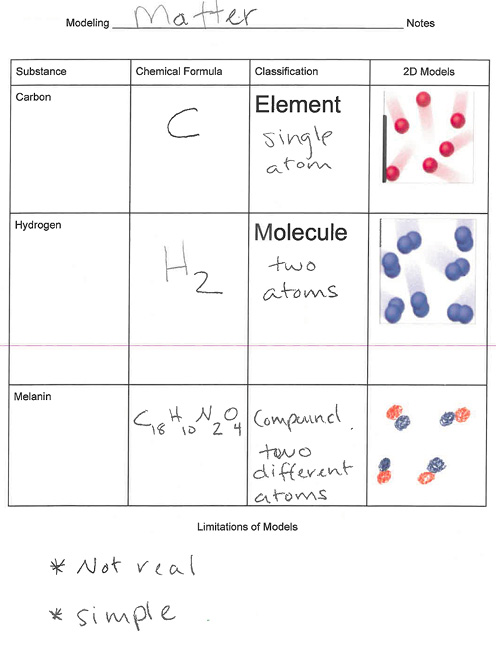
Exit assessment: Formula, classification, and model.
In their journals, students frequently pointed out that they heard about melanin before. It is a term that is frequently used in popular culture. They have heard and seen phrases such as “Your melanin is poppin” or #MelaninMagic. They were, however, unfamiliar with the chemical properties of melanin or the fact that melanin is found in plants and in nonhuman animals.
After the journaling, students completed a Daily Science Review, which is a five-question, multiple-choice assessment aimed at determining the degree to which students were able to differentiate between atoms, elements, molecules, and compounds. I use it to determine whether (and how much) I need to revisit the topics covered. The students did particularly well on the question assessing understanding of compounds, and the overall scores were notably higher than is typical of Daily Science Reviews.
Subsequent lessons build from this introductory lesson in two ways. First, students extend their knowledge of molecular modeling to describe and explain particle motion, density, temperature, and changes in states of matter as a function of adding or removing thermal energy. Second, students are presented with more complex molecules and compounds, as well as alternative ways to represent them.
Summary
The lesson implementation described here provides an example of how a socially transformative approach to science curriculum can be readily implemented in a traditional science classroom. The description also shows that subtle, yet powerful modifications proved beneficial to both my students and to me. The first readily identifiable benefit for my students was increased engagement. Students asked more questions, contributed more thoughtful input, and were more confident in themselves throughout the lesson. It was extended throughout the lesson and was evidenced in journal entries that (a) were more extensive, and (b) made more personal connections. A second benefit for my students was increased assertiveness. While students were more confident than normal throughout the lesson, when they were called on to critique the veracity of their models and of scientific models in general, they embraced that opportunity and asserted their ideas with vigor.
The modifications made using the socially transformative approach allowed me to include African Americans in my science curriculum and to be able to underscore something culturally affirming in doing so. •
Resources
PubChem—https://pubchem.ncbi.nlm.nih.gov/
Online Supplemental Resources
Daily Science Review—https://www.nsta.org/online-connections-science-scope
Jomo W. Mutegi (jmutegi@iupui.edu) is an associate professor in the Urban Teacher Education Department at Indiana University Purdue University at Indianapolis. Vanessa Gee is a doctoral student in the Urban Education Studies program at Indiana University Purdue University at Indianapolis and a science teacher in the Metropolitan School District of Washington Township in Indianapolis, Indiana.
Chemistry Equity Inclusion Instructional Materials Teaching Strategies Middle School


