Feature
Using Student-Generated Questions to Investigate Chemical Reactions
Materials
- 25 ml 0.1 M CuSO4 for each group (when preparing the solution, add a few drops of 1.0 M HCl or the reaction will not occur, Cl- disturbs the oxide layer on the aluminum allowing the Cu2+ to react with aluminum)
- Aluminum metal (small piece of wire and/or foil for each group)
- Beakers
- Stirring rods
- 25 ml graduated cylinder
Safety
Activities involve hazardous chemicals and require indirectly-vented chemical-splash goggles, nitrile gloves, and non-latex aprons. Use caution when handling glassware. Instructions for appropriate chemical disposal required must be provided.
Using student-generated questions to drive instruction is a valuable tool in the science classroom. Questions that arise while students make sense of phenomena lead to meaningful investigations where students engage in many science practices (Schwartz, Passmore, and Reiser 2017). Starting a unit or lesson by eliciting student ideas allows teachers to activate prior knowledge, help students represent their ideas, and identify ways to modify and structure further instruction (Windschitl, Thompson, and Braaten 2018).
In this lesson, students’ questions about the reaction between aqueous copper (II) sulfate and aluminum metal are used to drive an introduction to chemical reactions. While students begin the lesson by asking questions, they eventually engage in many of the science practices described in the Next Generation Science Standards (NGSS Lead States 2013). Students ask questions about the reaction, plan and carry out investigations to understand the components of the reaction, interpret data, construct explanations, and engage in argument from evidence using the Claim-Evidence-Reasoning method. This lesson was taught in chemistry classes and includes modifications to meet the needs of diverse learners. As described, it requires four or five instructional days. However, the lesson could be modified and reduced to two or three instructional days if some parts of the lesson are modeled by the instructor in the interest of time. This lesson focuses on the reaction of copper (II) sulfate and aluminum and describes other specific chemical reactions, but it could be modified to focus on different chemical reactions depending on the materials available. The goal is for students to engage in chemistry through their own observations and questions, which can be achieved using chemical reactions other than the ones described here.
Introducing the Phenomenon and Asking Questions
Start class by asking students about their experiences with chemical reactions. Explain that your class is starting a unit on chemical reactions with the following goal: every student will engage with a chemical reaction, make observations, and ask questions about what is happening. Emphasize that the students should make detailed observations, which will help them figure out what is happening.
The investigative phenomenon for this lesson is the reaction between aqueous copper (II) sulfate and aluminum metal (see Figure 1 for results of reaction).
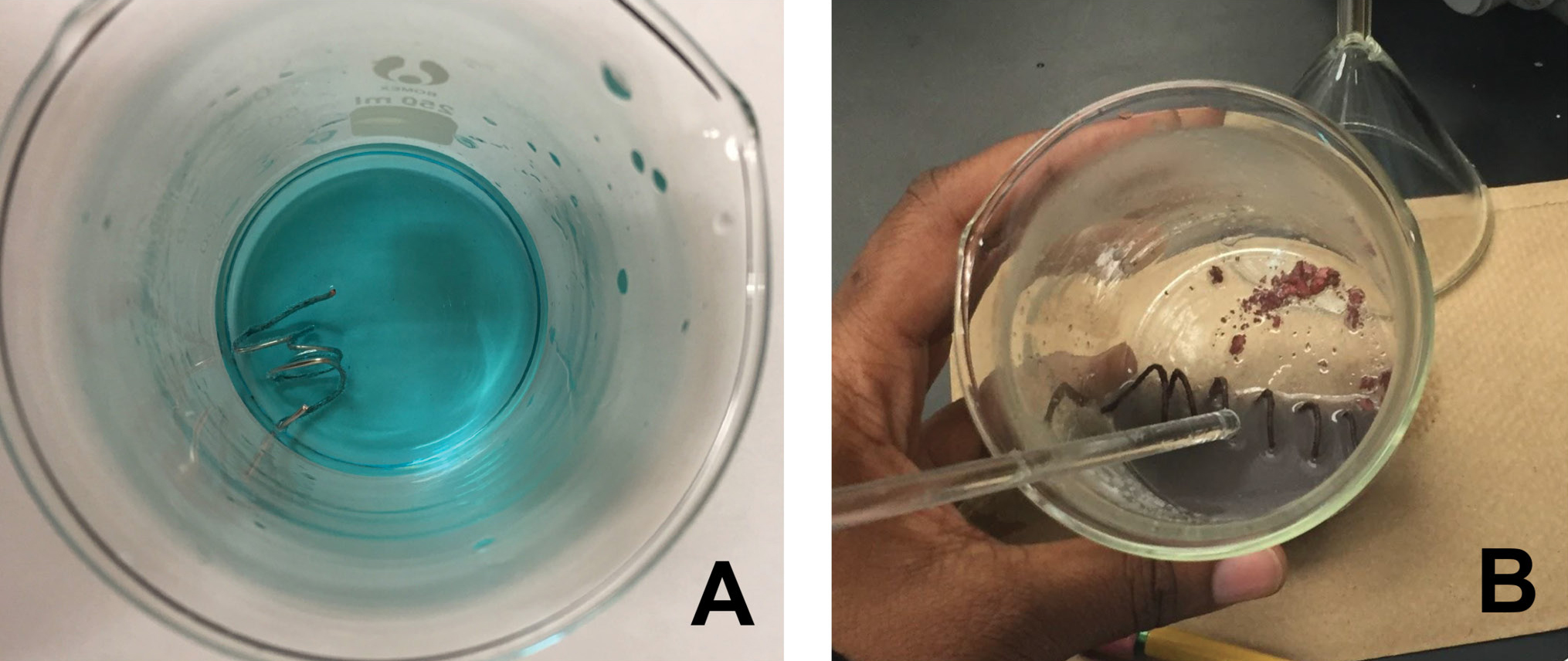
Aqueous copper (II) sulfate and aluminum wire just after mixing (A) and after the reaction has proceeded (B).
Before starting the lab, review safety expectations with students. Students must wear goggles and aprons throughout the lab and dispose of waste in a waste container, not in the sink. The solid waste and solution should be separated by decanting and/or filtering. The solid waste can be disposed of in the trash, and solutions can be disposed of in the sink with plenty of water.
After reviewing safety expectations, students divide into lab groups of three to four students and receive the following materials and instructions (see Supplementary Resource 1). Students should be grouped to align with the teacher’s goals for this activity, keeping in mind that intentional student groupings are a useful tool for differentiation. The materials should be prepared prior to class and available at each lab station. To reduce the use of chemicals, this reaction could also be performed in a test tube using smaller quantities.
Procedure
- Measure 25.0 ml of 0.1 M copper (II) sulfate, CuSO4, and pour it into a beaker.
- Add aluminum to the copper (II) sulfate solution (you can use aluminum foil, aluminum wire, or both). Stir the aluminum around in the solution.
- Record any observations. Be detailed! This will be useful information later.
- Dispose of everything in your reaction beaker in the “waste container.”
Students share their observations with the class and create a class list of observations about the reaction. This discussion should focus on observations not explanations; encourage students to use language they are comfortable with. Some examples of student observations are:
- “the aluminum foil disappeared,”
- “the blue color went away,” and
- “reddish or brown stuff formed.”
Most students jump to the conclusion that the red/brown stuff is rust. To begin addressing this misconception, students answer two questions on their own (see below), then discuss with their partner or group.
- Many students think that the “red/brown stuff” looks like rust (Fe2O3). What data could you collect in the lab to investigate the claim that the “red/brown stuff” is rust?
- What are the reactants in this chemical reaction? Do you have any ideas about what the products could be?
These questions help students think about what they already know about chemical reactions, and physical and chemical properties.
Next, each student makes a list of scientific questions about the reaction they observed (4–5 questions per student is ideal). Explain to students that their questions are important because they are going to use their questions to drive additional investigations on chemical reactions. Schwartz and colleagues recommend using the starter, “Why does…?” to help students develop meaningful explanatory questions they can pursue via investigation (Schwartz, Passmore, and Reiser 2017). Students can submit their questions on paper or sticky notes, or use a Google form (which will help if you want to print them).
What should we investigate?
Prior to the next class, read the students’ questions. Students’ questions generally fit into six categories:
- Questions about the solution
- Questions about the metal
- Questions about the red/brown stuff
- Questions about the process of a chemical reaction
- Questions about the speed/rate of the reaction
- Other questions
Prepare chart paper or posters labeled with these categories (or other categories, depending on your students’ questions) and hang them around the room. Prepare envelopes containing about 10 of the students’ questions from the day before—make sure they’re mixed up so each envelope contains a variety of questions. Distribute an envelope of questions to each student group. Explain to students that you noticed their questions fit into several main categories, so they should organize the questions by category to plan their investigations. Students read their assigned questions, copy them onto a sticky note, and place them on the poster in the category where the question best fits (Figure 2). Examples of student questions include the following:

Student questions placed on the poster.
- Why does the aluminum foil disappear?
- Is the new substance that was made rust (iron (III) oxide)?
- Why does the blue color disappear?
- What if we used a different metal other than aluminum?
- Could you mix the aluminum with a chemical compound solution that wouldn’t make it dissolve?
Students observed that many of their peers also had questions about the type of solution, the type of metal, and the red/brown stuff. Lead a class discussion about how the students could use these questions to drive the next investigation and figure out what the red/brown stuff is. As students find the answers to these questions through investigation and analysis, they discover that atoms are conserved in chemical reactions and chemical reactions happen in predictable ways. The work students do to answer their own questions guides them toward mastery of the disciplinary core idea PS1-B (The fact that atoms are conserved, together with knowledge of the chemical properties of the elements involved, can be used to describe and predict chemical reactions).
The class’s guiding questions became “What is the red/brown stuff?” and “What is happening to produce it?” Students determined that answering many of their other questions would help them answer these guiding questions. When this lesson was first taught, only the first question was used (What is the red/brown stuff?), but the addition of a second question (What is happening to produce it?) now requires students to produce more detailed explanations. It is important for students’ questions to remain on display so students can go back to the questions and determine what they have answered, what they still need to figure out, and what new questions they have (Schwartz, Passmore, and Reiser 2017).
Before moving on, students need to become familiar with the types of chemical reactions and how to use patterns to predict products. Students receive several chemical reactions on strips of paper (see Supplementary Resource 2) and sort them into groups based on patterns and similarities (Figure 3). Students match each group of reactions with a name (synthesis, decomposition, single replacement, double replacement, and combustion) and write a definition for that type of reaction (see Supplementary Resource 2 for sample student answers). Students make general observations about the reactions—they notice that the atoms on the reactant side and the product side are the same, just rearranged. This sorting activity gives students background knowledge on the types of chemical reactions and how patterns are used to analyze reactions and predict products.
Planning and Carrying Out Investigations
Remind students about their questions about the reaction between copper (II) sulfate and aluminum. Tell them that there were many interesting questions about the type of solution and the type of metal, and they will use those questions to plan and carry out two investigations. Some of the questions students have about these topics include
- Would we get the same reaction if we put the aluminum in a different solution?
- Why did the solution change color?
- What would happen if we combined different metals with the copper (II) sulfate?
- Why did the aluminum dissolve?
Before beginning the investigations, review safety expectations with students. In an effort to reduce the use of chemicals, these reactions could also be performed in test tubes using smaller quantities.
Investigation 1: What happens if you use a different solution?
Materials:
- 0.1 M FeCl3
- 0.1 M CuCl2
- 0.1 M CaCl2
- Aluminum wire or foil
- Beakers
- Graduated cylinder
- Stirring rod
In the first investigation, students figure out what happens when different solutions are used. To investigate the role of the solution in the reaction, students design an investigation using 0.1 M iron (III) chloride (FeCl3), copper (II) chloride (CuCl2), and calcium chloride (CaCl2) solutions and aluminum metal. Students discover that the “red/brown” stuff only forms when the CuCl2 solution is used (Figure 4).
Investigation 2: What happens if you use a different metal?
Materials
- 0.1 M CuSO4
- Magnesium ribbon
- Zinc pieces
- Beakers
- Graduated cylinder
- Stirring rod
In the second investigation, students figure out what happens when different metals are used. To investigate the role of the metal in the reaction, students design an investigation using CuSO4 solution and zinc and magnesium metals. Students discover that the reaction of CuSO4 with both zinc and magnesium also produces the “red/brown” stuff, just like with aluminum (Figure 5). See student resource (Supplementary Resource 3).
Sorting chemical reactions.
Instructions:
- Compare and contrast each of the reactions and put the reactions in groups according to similarities and patterns you notice. There are five possible groups.
Hints:
- Look at how many reactants there are and what types of elements/compounds the reactants are.
- Look at how many products there are and what types of elements/compounds the products are.
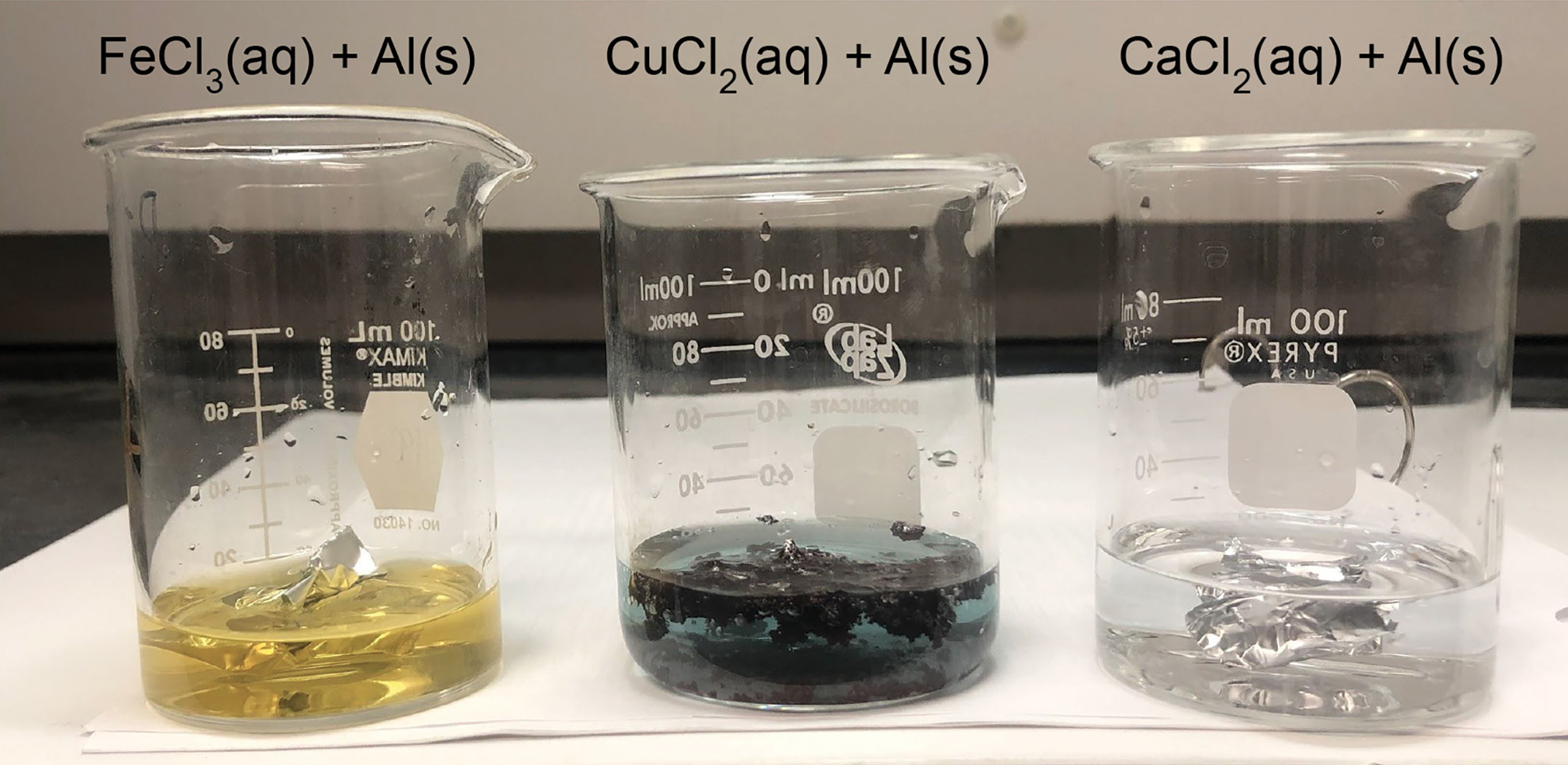
What happens if you use a different solution?

What happens if you use a different metal?
Students analyze their results with their lab groups (Figure 6) and quickly realize that the only combination of metals/solutions that produced the “red/brown stuff” contained copper ions in the solution. They conclude that the red/brown stuff must be copper.

Students analyzing results in their lab groups.
Next, students review their original questions about the type of metal, the type of solution, and the red/brown stuff and identify which questions they can answer now. Students spend some time in groups discussing answers to these questions. As a quick formative assessment, students answer two questions as an exit ticket:
- What is still confusing to you?
- What else do you want to know to support your claim about the red/brown stuff?
This will help identify any continuing misconceptions that can be addressed during the next class. Depending on the results, you may choose to have students conduct additional investigations, go over the results from the investigations in more detail, or develop scaffolds to help students reach a more detailed understanding of the reactions.
Claim, Evidence, Reasoning
In the final activity, students use the information and data they collected to create a scientific argument. Students provide a clear claim, support it with evidence from the investigations and classroom activities about chemical reactions, and provide a reasoning that uses scientific concepts to connect the claim and evidence. Students were encouraged to include diagrams or sketches to model their thinking about the problem (students in this class had extensive previous experience with modeling and particle level diagrams). They were also provided with sentence starters, questions, or other prompts to guide their writing. Opportunities for modeling and prompts to guide writing allowed all students to demonstrate understanding of the chemical reactions, which ensured that all students were engaged in the sense-making process.
Once students have created their CER, they share ideas with peers through an argumentation session (Sampson, Grooms, and Walker 2009). Finally, students revise their arguments based on discussions and feedback. Figure 7 shows the “criteria for success” developed by students before working on their CER, Figure 8 shows an example of a CER, and Figure 9 shows the rubric used to evaluate students’ work (see Online Connections).
Criteria for success.
Your scientific argument must include:
- A clear answer to the guiding question (claim)
- Evidence from each part of the investigation
- Information about the type of reaction occurring
- Information about the reactants and the products in each reaction (or nonreaction)
- Pictures/diagrams to help explain processes
- Information about why the red/brown stuff can’t be rust
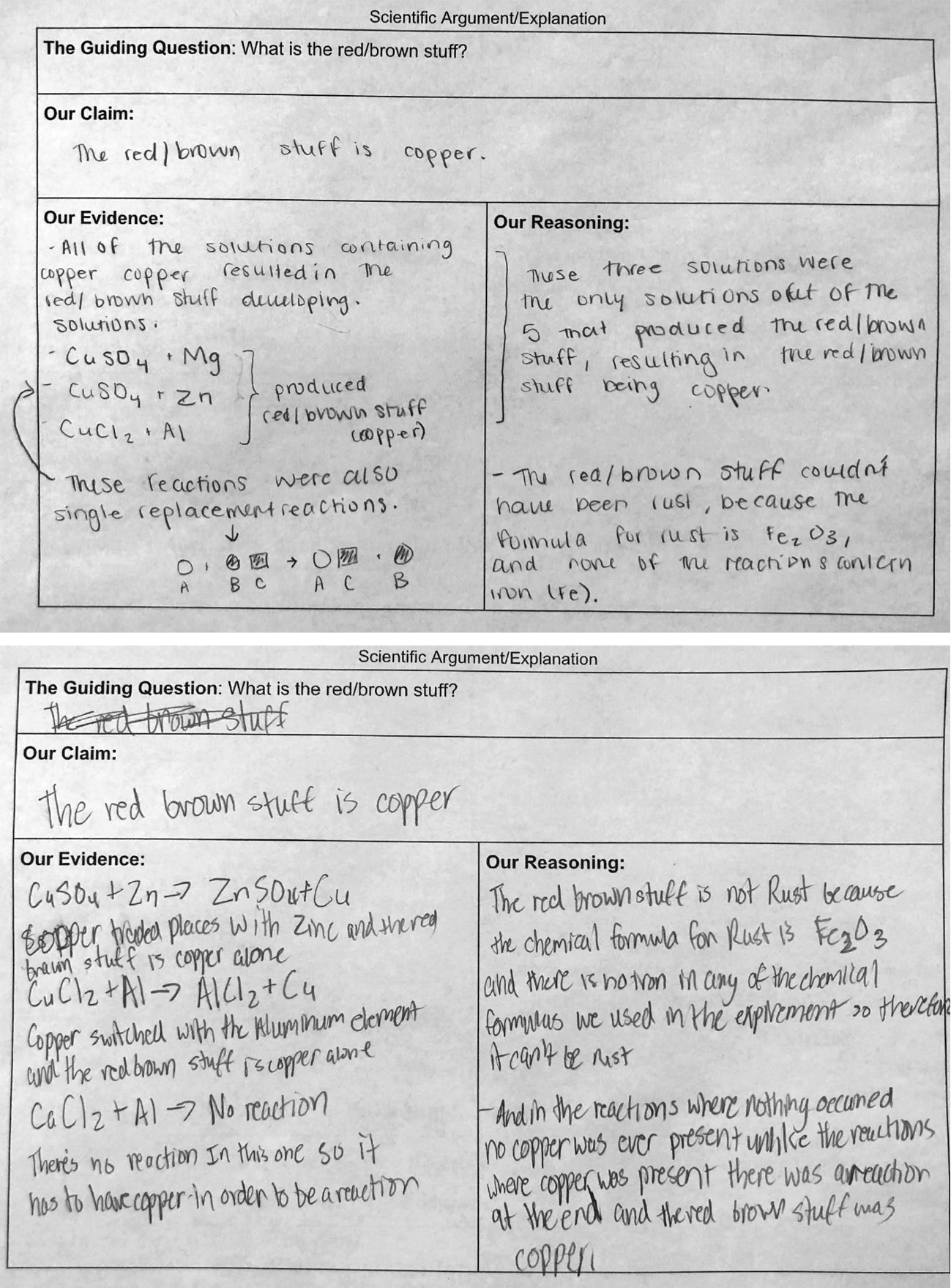
Examples of CERs.
Conclusion and Opportunities for Extension
Providing students with the opportunity to investigate their own questions was a valuable way to introduce chemical reactions. Allowing every student to engage with the phenomenon and ask questions about it provided an “on ramp” for all students to find interest and success. All these investigations could be done without eliciting student questions and ideas, but students were excited to investigate their own questions. This lesson is well-suited for differentiation and can be easily modified to meet the needs of diverse learners. Throughout the lesson, students might require or benefit from additional scaffolds such as sentence starters for asking questions, outlines for planning investigations, and support in developing their explanations and scientific argument.
Because of the scope of this lesson, unfortunately many students’ questions about the investigative phenomenon were not fully answered. However, there are numerous opportunities for extensions. Students could explore some of their other questions, even if they might not relate directly to the purpose of this lesson. Students could explore the activity series and test several other metals/solutions, investigate the kinetics of reactions, or start making sense of balanced reactions and stoichiometric relationships—there are a lot of interesting chemical ideas for them to investigate! Students’ arguments would be improved if they compared the physical and chemical properties of rust and copper to the red/brown stuff that was produced in the reaction. ■
Online connections
Resource 1—Copper Aluminum lab: https://www.nsta.org/sites/default/files/journal-articles/TST88-2/Gawne/Resource_1__Copper_Aluminum_lab.docx
Resource 2—Reactions and sample student answers: https://www.nsta.org/sites/default/files/journal-articles/TST88-2/Gawne/Resource_2__reactions_for_sorting.docx
Resource 3—What is the red/brown stuff? https://www.nsta.org/sites/default/files/journal-articles/TST88-2/Gawne/Resource_3__what_is_the_red_brown_stuff_.docx
Rubric: https://www.nsta.org/sites/default/files/journal-articles/TST88-2/Gawne/Figure_9_Rubric.pdf
Hillary Gawne (hillary.gawne@gmail.com) is a chemistry teacher at Northwood High School in Silver Spring, Maryland
Chemistry Labs NGSS Science and Engineering Practices High School



