feature
Reflections on Multimodal Delivery of a Laboratory Course for Nonscience Majors and Opportunities for Improved Student Engagement
Journal of College Science Teaching—September/October 2022 (Volume 52, Issue 1)
By Mark Vincent dela Cerna
A rudimentary level of scientific literacy is necessary in the general public. At the undergraduate level, this literacy can be achieved through general education courses offered in areas of natural sciences. Over the past several years, practical courses have been developed to make the teaching of chemistry concepts in the laboratory more interesting and appealing to nonscience majors. This article provides insights and reflections from teaching a general education, liberal arts chemistry course at a private university using different teaching delivery modes during a pandemic. Specifically, the unique circumstances of the COVID-19 pandemic allowed faculty to deviate from the traditional face-to-face delivery and explore the use of virtual delivery, experimentation at home, and hybrid instruction to meet learning objectives. The article reflects on the lessons from this experience to improve course delivery and student engagement in science laboratory courses for nonscience majors.
The need for increased scientific literacy in the general public is paramount, especially with current issues across the globe, such as climate change, the ongoing COVID-19 pandemic, and advances in human genome editing, among others (Eilks et al., 2014; Maiennschein, 1998; Fatimah & Anggrisia, 2018; Anderson et al., 2020; Van Eijck, 2010). An understanding of basic scientific principles and the ability to critically evaluate facts enrich public discourse about advances in these fields and others and are relevant for developing policies that will guide further developments and applications in many areas of research (Eilks et al., 2014; Van Eijck, 2010).
At the college level, increasing scientific literacy is partially addressed by introducing fundamental scientific principles through general education courses in the natural sciences, allowing faculty from natural sciences departments to engage nonscience majors (Tro, 2004). For instance, introductory chemistry courses that focus on practical aspects such as those relating to the environment, food, or fragrance have been designed to make chemistry more appealing (Logan & Rumbaugh, 2012; Miles & Bachman, 2009; Uffelman, 2007; Kaplan, 1993; Neuman & Harmon, 2019). In addition to engaging students, however, the concepts covered in natural science general education courses must also be related to issues that are universally relevant to humanity. The goal of a liberal arts education is, in part, to prepare students for citizenship and life (Tro, 2004). An interdisciplinary course is arguably suitable for advancing scientific literacy because it allows students to borrow concepts from several disciplines and apply them to diverging problems. For instance, a biophysics course can connect fundamental principles to applied areas such as health (Parthasarathy, 2015).
In this article, I reflect on our experience of delivering a liberal arts chemistry laboratory course at a private university with a Carnegie Classification as a doctoral/professional university. This course is among many across the globe that has been impacted by the COVID-19 pandemic, so it provided the opportunity to explore different modes of delivery of instruction.
Liberal arts chemistry course format
The liberal arts chemistry laboratory course is traditionally offered as a 2-hour, in-person course over the span of 12 weeks, with students exploring various chemistry concepts in an undergraduate chemistry teaching laboratory. In response to COVID-19 guidelines limiting laboratory occupancy, the class was designed to be hybrid flexible (HyFlex). Furthermore, following university policies, accommodations were made for students who had to be completely remote throughout the semester. This put those of us teaching the class in a unique situation of teaching a single course in two formats: HyFlex and remote. In this article, I focus on lessons learned from the remote class. The primary challenge of this dual classroom is maintaining the quality of instruction and meeting learning objectives regardless of how a student attends the class. As the same concepts were covered for both groups, several modes of instruction (see Figure 1, Table 1, and Online Appendix) had to be used to meet students’ needs and the demands of the experiments.
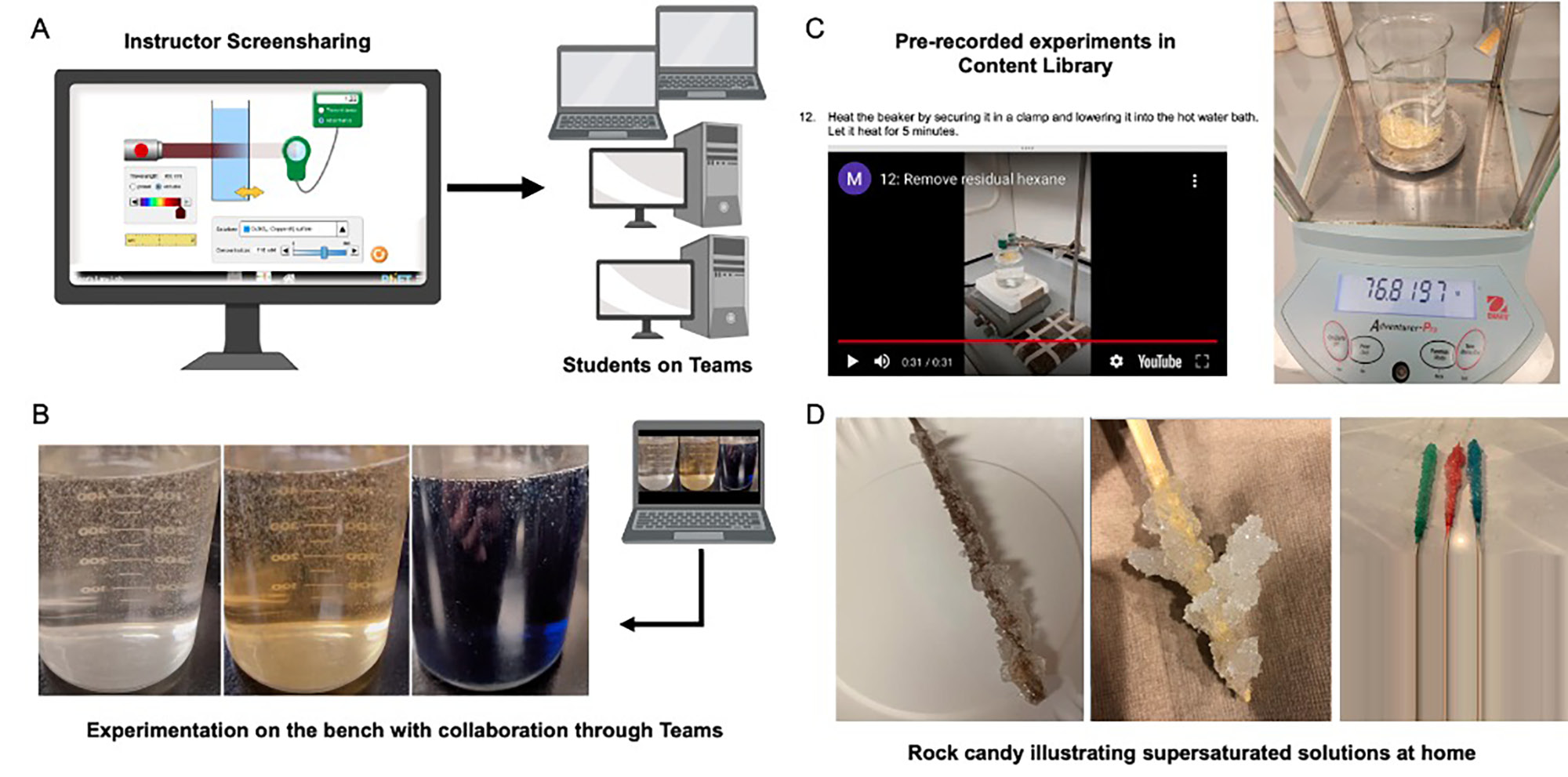
Representative activities in various delivery modes.
Note. Simulated labs included use of BeyondLabz and browser-based activities. Demonstration of Beer’s law was done using the University of Colorado’s PhET simulations combining guided discussions with instructor screensharing on Microsoft Teams and individual student exploration (A). Completely remote students were involved in preparing solutions for the Briggs-Rauscher reaction as they worked in tandem with their HyFlex counterparts who hosted their own meetings on Teams on their benches (B). Determination of the fat content of chips was prerecorded, with videos and images accompanying each step. All measurements were included in photos to allow students to do their own data collection and analysis (C). This image shows representative results, with varying success, from the rock candy experiment, which was used to demonstrate supersaturated solutions and crystallization at home (D).
| Table 1. Four modes of delivery of instruction to the completely remote group. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Bridging gaps through multimodal instruction
Hands-on laboratory exercises are crucial to the experience of chemistry students (Sansom & Walker, 2020). Unfortunately, this format is the first to be sacrificed in the shift to online learning. For this reason, providing opportunities for students to perform their own experiments and analyses was critical when designing activities for the class. Laboratory courses have at least three aims: (a) imparting a specific set of skills, including observation, experimentation, and interpretation; (b) encouraging critical thinking; and (c) allowing students to experience phenomena firsthand (Seery et al., 2018; Reid & Shah, 2007; Abraham et al., 1997). When engaging nonscience majors, the third goal is perhaps most important. To address these goals, we needed to use a combination of different instruction delivery modes.
Three of the four modes of delivery (see Table 1)—namely simulated labs, livestream, and home experiments—address this goal by requiring live participation from the remote students. Recorded experiments require the least engagement to perform the experiment but do require an understanding of the procedure to analyze the collected data.
Simulated labs were used for critical experiments that cannot be replicated at home due to demands for proper equipment and glassware or a controlled setting such as the laboratory; examples of such activities include the use of lasers for a physics lab, animal dissection for a biology lab, or the use of spectrometers for a chemistry lab. Simulated labs, whether through the use of dedicated software or web-based activities, can be reliable alternatives to physical labs and, when properly delivered, can improve student performance and attitude (Corter et al., 2007; Son, 2016). We used a simulated laboratory to perform acid-base titration and a web-based tool to explore Beer’s law (see Online Appendix). The simulated labs were most challenging for students who did not have any prior laboratory experience. However, students also noted that these labs were less intimidating because they knew mistakes would not lead to irreversible consequences and that they were more likely to explore in the lab. On one hand, that students have confidence in finding their own way is a positive aspect of simulated labs, but the instructor needs to emphasize proper techniques by providing demonstrations or supplemental pre-recorded discussions.
Livestreaming allows remote students to participate in class and engage the instructor and their peers. This mode has the potential to encourage collaboration among students when a student in the face-to-face format and a remote student are paired together (Figure 1B). The caveat is that students have to be provided with laptops to host a video call or be allowed to use personal computers in the laboratory. With such pairings, planning can be done together, and experiment tasks are split, with the face-to-face student performing the tasks while the remote student takes notes. Time can then be provided at the end for collaborative discussion and analysis. In our lab, we provided students a basic protocol for an oscillating reaction, the Briggs-Rauscher reaction. Pairs were then tasked with exploring the effects of varying reactant volumes and starch types. This activity required communication among pairs, which resulted in improved engagement (Laredo, 2013; Laal et al., 2013). Demonstrations can also be done via livestreaming, especially at the start of a session, to capture student attention, such as by performing the “burning dollar” demonstration or the colorless-pink transition of phenolphthalein.
These two modes of delivery are enabled by technology through the use of virtual reality laboratories, simulations, or video-conferencing and livestreaming. Previous studies note that students engage in and learn from these technology-enabled formats but still prefer hands-on experience (Corter et al., 2007). Indeed, while students are engaging in the phenomena in these modes, they still miss the true hands-on experience.
To address the third goal further, we utilized experiments and demonstrations that students can execute at home. When designing these activities, the instructor needs to be fully aware of resources available to remote students. Moreover, as much as possible, out-of-pocket costs must be kept at a minimum, or to virtually nothing, and no hazardous chemicals or substances that require special disposal protocols should be involved. Most students will have access to a kitchen, and technology for remote students is also expected. Access to a kitchen and technology open up significant opportunities for designing hands-on experiments. Several “kitchen science” activities in physics, chemistry, and biology have been designed and executed (Vieyra et al., 2017; Gya & Bjune, 2021; Nguyen & Keuseman, 2020; Schultz et al., 2020; Andrews et al., 2020). A properly designed at-home experiment should provide the student the opportunity to use alternative materials, have independent variables that can be explored, and be able to be tied back to a concept or laboratory exercise. In our course, students were tasked with capturing and quantifying carbon dioxide released by dissolving Pop Rocks in soft drinks using a balloon (see Online Appendix). The “Pop Rocks and Coke” myth was featured in MythBusters, and the episode was used to pique students’ interest. Without prompting, students explored variables such as type of soft drink and temperature. The experiment also stimulated a discussion on ideal gases, solubility, production of Pop Rocks, and revisiting the conclusions from the MythBusters episode. In our experience, experiments delivered through this mode are the most successful based on student feedback and participation.
The fourth mode is the use of recordings. This mode has the least potential for active engagement during the experiment but requires students to understand the procedure to answer postlaboratory questions. This mode is a last resort and was only utilized once in our course during a quarantine period (see Online Appendix). Recordings can, however be helpful for demonstrating proper techniques and use of equipment and can supplement student learning as needed. Because recordings are delivered asynchronously, they can best be used as resources for students in reviewing best practices and proper use of instruments and equipment or in tandem with simulated labs.
Interesting phenomena, collaborative learning experiences, and exploratory work
To improve scientific literacy in the general public, significant changes must be made at all levels of education and in how science is communicated by practitioners to the public (Fatimah & Anggrisia, 2018; Anderson et al., 2020; Van Eijck, 2010). One way to improve scientific literacy is to better engage nonscience majors at the college level through natural science electives. Student exploration and problem-solving opportunities in a laboratory course can result in improved outcomes and student understanding (Laredo, 2013; Walker et al., 2012).
Our experience during the COVID-19 pandemic provided us the opportunity to explore various modes of delivery of instruction, as discussed in the previous section. Lessons from these modes’ utility in engaging students and meeting course objectives, however, provide valuable insight for how to design and develop experiments postpandemic so we can improve the way these natural sciences courses engage nonscience majors.
Activities that begin with student engagement, are supplemented by opportunities for knowledge building, and culminate in exploration and discovery should enrich the student experience and improve performance, understanding, and retention (Figure 2). We illustrate this using the classic acid-base titrations chemistry experiment (Figure 2B), but the general strategy should be applicable to other natural sciences.
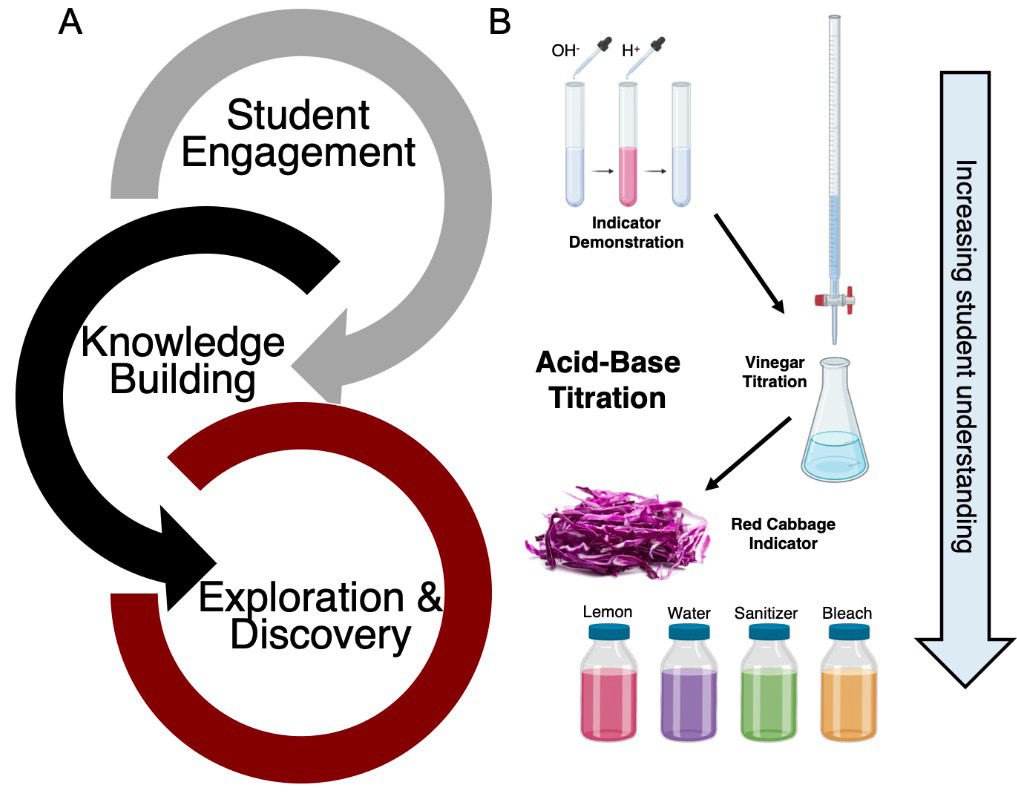
General strategy to improve student learning.
Note. An integrated approach of student engagement, knowledge building, and exploration and discovery can be integrated within a single experiment or a series of experiments in the laboratory (A). One scenario for applying this approach using the classic acid-base titration experiment can involve a demonstration of reversibility of pH indicators (engage), a vinegar titration experiment in the laboratory (build), and a homemade red cabbage indicator to test the pH of household items (explore), which will increase student understanding of the concept (B). Part of the figure was created with Biorender (https://www.biorender.com).
Through face-to-face delivery or via livestreaming, the instructor can demonstrate the colorless-pink transition of phenolphthalein to capture students’ attention. This demonstration can be done at the beginning of a session or a segment. Well-executed demonstrations draw students’ attention, and unexpected or perplexing results can be a starting point for inquiry and learning (Meyer et al., 2003). Mixing colorless liquids (acid and base) and yielding a pink solution is “almost magical,” and the reversibility of the phenomenon further adds to students’ fascination. Knowledge building is then done by discussing pH indicators and performing classical titration experiments in the laboratory or with simulated labs. Knowledge building should include discussion of not only concepts but also proper measurements, laboratory etiquette, and other topics. If possible, a relatable angle can improve the exercise by allowing the student to take on the role of an investigator verifying the acetic acid content of commercial vinegar. Finally, a kitchen experiment can be assigned in which students create their own pH indicators from red cabbage and investigate solutions available to them at home. This sequence incorporates several modes of instruction delivery, builds on the natural interest of students, and includes guided experimentation (classic titration) that leads to independent investigation. A similar sequence is possible for other experiments and concepts.
Realistically, it is probable that social distancing will remain part of the norm in the future, and remote components will be critical to the success of laboratory courses. Thus, it is crucial for instructors to incorporate the Engage-Build-Explore model (Figure 2) into a combined approach in which guided, structured exercises are supplemented with exploratory activities at home. This approach can be enhanced by relating lessons to contexts relevant to students’ daily lives. While this hybrid, multimodal approach is critical during times of social distancing, the strategy should be equally applicable under “normal” circumstances and should still be explored postpandemic.
Conclusion
Overall, these approaches reveal significant opportunities to improve laboratory courses delivered to liberal arts or nonscience majors. Such approaches use a simple reorganization of experiments and demonstrations and take advantage of the apparently innate synergy between face-to-face traditional laboratory experiments and remote learning using simulations and home experiments.
Mark Vincent dela Cerna (mdelacerna@georgiasouthern.edu) is an assistant professor in the Department of Chemistry and Biochemistry at Georgia Southern University in Savannah, Georgia.
General Science Interdisciplinary Teacher Preparation Teaching Strategies


