feature
Airbags as Real-Life Applications for Science
At the core of an effective model-based inquiry (MBI) unit is a scientifically rich, complex phenomenon that serves as the reason for engagement and drives student sensemaking and investigations throughout the unit. Engaging in iterative attempts to explain phenomena over the course of an MBI unit allows the opportunity for students to construct evidence-based explanations that are refined over time with various science practices. We provide an example of using MBI as an instructional model to facilitate students’ conceptual understanding of chemical reactions.
MBI is a framework utilized for designing science units focused on the construction, revision, and testing of models by students with aims of developing explanations of natural phenomena (“What is MBI?” 2020). While traditional science units offer a series of activities centered around a particular topic summatively assessed at the end of the unit, MBI units are driven by the desire to explain anchoring phenomena. The MBI unit then culminates with individual evidence-based explanations of the anchoring phenomenon.
The focus of the MBI unit described here is chemical reactions. Chemical reactions are responsible for saving lives every day. According to the National Highway Traffic Safety Administration (NHTSA), airbags, combined with the use of seatbelts, are considered to be the most effective safety protection available for vehicle occupants (“Facts + Statistics” 2020). NHTSA estimates that the combined use of airbags and seatbelts reduces risk of death in frontal crashes by 61%, compared to a 50% reduction by use of seatbelts alone, and a 34% reduction by use of airbags alone. Dating back to 1999, all vehicles are required to have driver and passenger frontal airbags installed (“Airbags” 2020). Frontal airbags are said to be responsible for saving an estimated 2,800 vehicle occupants ages 13 and up in 2017.
Although airbags serve the purpose of protecting vehicle occupants in the event of a crash, faulty airbags can result in an increased risk of injury during a collision. For example, from 2015 to 2019, an estimated 70 million vehicles were recalled due to faulty Takata airbags (“Airbags” 2020). The faulty design resulted in fifteen deaths and more than 250 reported injuries, and precipitated the largest vehicle recall to ever occur in the United States. Given these statistics, it is evident that proper airbag design and safety is essential in reducing risk of injury and death in vehicle collisions.
Unit description
Over the course of 14 days, in 50-minute class periods (see Online Resources, Table 1), high school chemistry students developed a deep conceptual understanding of chemical reactions and the role they play in airbag design and safety through a variety of investigations and activities aligned to the Next Generation Science Standards (see Online Resources, Table 2). Students were then able to apply their knowledge to an airbag engineering design activity. To focus on the engineering design aspect of this activity, we recommend this task be used as the culminating activity to a unit on chemical reactions. However, since the airbag phenomenon applies to many different areas of science, this unit and engineering design task can be modified to suit additional units such as a high school chemistry unit on gas laws or a high school physics unit on momentum.
We recommend that before students attempt this engineering design task, they should be familiar with terms such as reactant, product, law of conservation of mass, limiting reactant, excess reactant, theoretical yield, actual yield, and percent yield; be able to balance chemical equations; be able to perform relevant stoichiometric calculations; and have appropriate laboratory skills.
Model development
Developing and using models is one of the core science and engineering practices within NGSS (NGSS Lead States 2013). When we use modeling in a science classroom, we initiate an iterative cycle of creating, revising, and testing our models against the real world. These models typically include a combination of pictorial representations and written explanations about observable and unobservable processes describing how and why a particular phenomenon occurs. Importantly, modeling allows for the opportunity to make students’ thinking visible to not only themselves, but to their peers and their teacher. It is important to recognize that when we talk about modeling in the science classroom, we think of models as tools for sensemaking throughout a unit, rather than thinking of them as products of a unit.
In day 1 and day 2 of this MBI unit, we introduce students to the anchoring phenomenon (i.e., airbags) through a series of simulated crash videos depicting the process of airbags deploying (see Online Resources). The goal of these videos is to elicit student observations and initial hypotheses about airbag systems. During this beginning stage, we pose the driving question of the unit: How can we design an airbag to inflate with the right amount of gas to prevent harm? Based on their initial observations and hypotheses, small groups of two or three students create initial models to represent airbag systems. Their initial hypotheses (Figure 1) are used as a public record throughout the unit.

Initial hypotheses developed by students.
Constructing initial models gives the opportunity to uncover students’ prior knowledge and initial thinking about the phenomenon presented. In this unit, students focus more on pictorial representations (Figure 2) than written explanations when constructing their models. Students may need additional guidance on representing the unobservable mechanisms within the phenomenon (i.e., what is happening inside the airbag that we can’t see). Recognizing the role unobservable factors play in the phenomenon is crucial for helping students develop a conceptual understanding of why the observable mechanisms occur.
A fundamental part of MBI is supporting students’ ongoing changes in thinking. To help students recognize their changes in thinking, we allow them to revise their initial models on day 7, halfway through the unit. Up to this point, students focused on physical and chemical changes, the law of conservation of Mass, types of chemical reactions, and how to balance chemical equations. To keep their thinking visible, students add to a summary table after each activity or investigation (see Online Resources, Table 4). We wanted students to use this new information to make stronger connections in their models about the role chemical reactions play in airbag systems.
Students are also given the opportunity to revise their model by adding sticky notes to their initial models (Figure 3), addressing new information learned, or questions concerning parts of the model that still lacked evidence. Additionally, students participate in a gallery walk to review other models and can leave sticky notes with comments or suggestions related to revising the model. This is a great opportunity for students to use other students’ thinking as a resource for learning. While we acknowledge changes in student thinking are ongoing throughout the entire unit, we don’t recommend providing numerous opportunities for model revisions in order to avoid generating “model fatigue” (Windschitl, Thompson, and Braaten 2018). Therefore, we opted to provide one opportunity for revisions halfway through the unit.
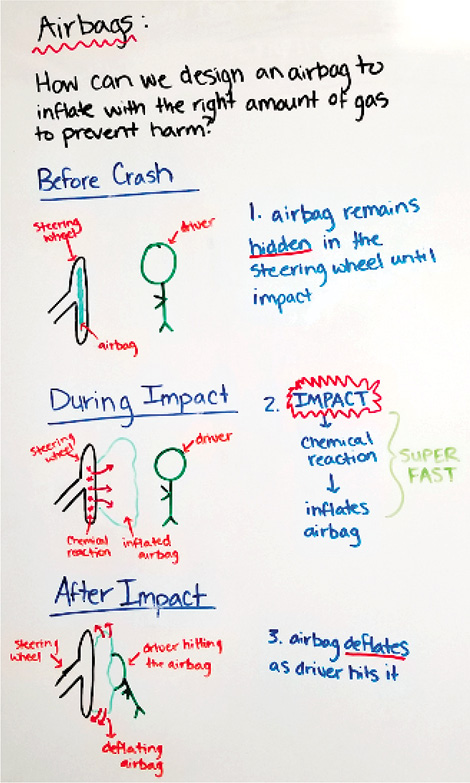
Example of initial model.
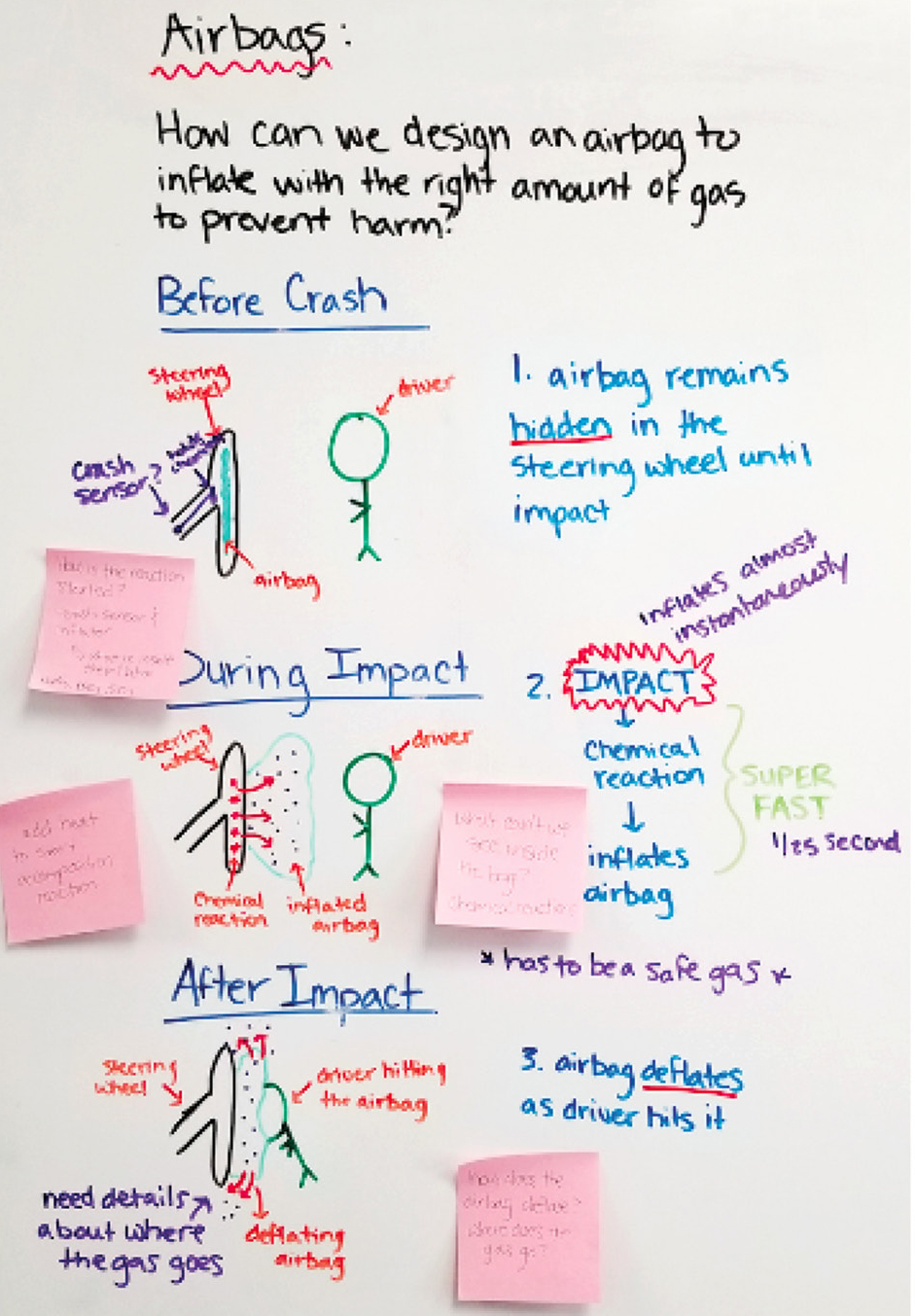
Example of revised model.
Another fundamental part of MBI is pressing for evidence-based explanations. This concluding stage of MBI consists of finalizing student models, building a consensus around student models and constructing a gotta-have-checklist before writing individual evidence-based explanations. On day 14, students refer back to public records (i.e., summary tables) and learning experiences throughout the unit to finalize their models (Figure 4). To help build a consensus around student models, the whole class produces a gotta-have-checklist (Figure 5) to serve as an outline for what would be necessary to include or expand upon in the final models.
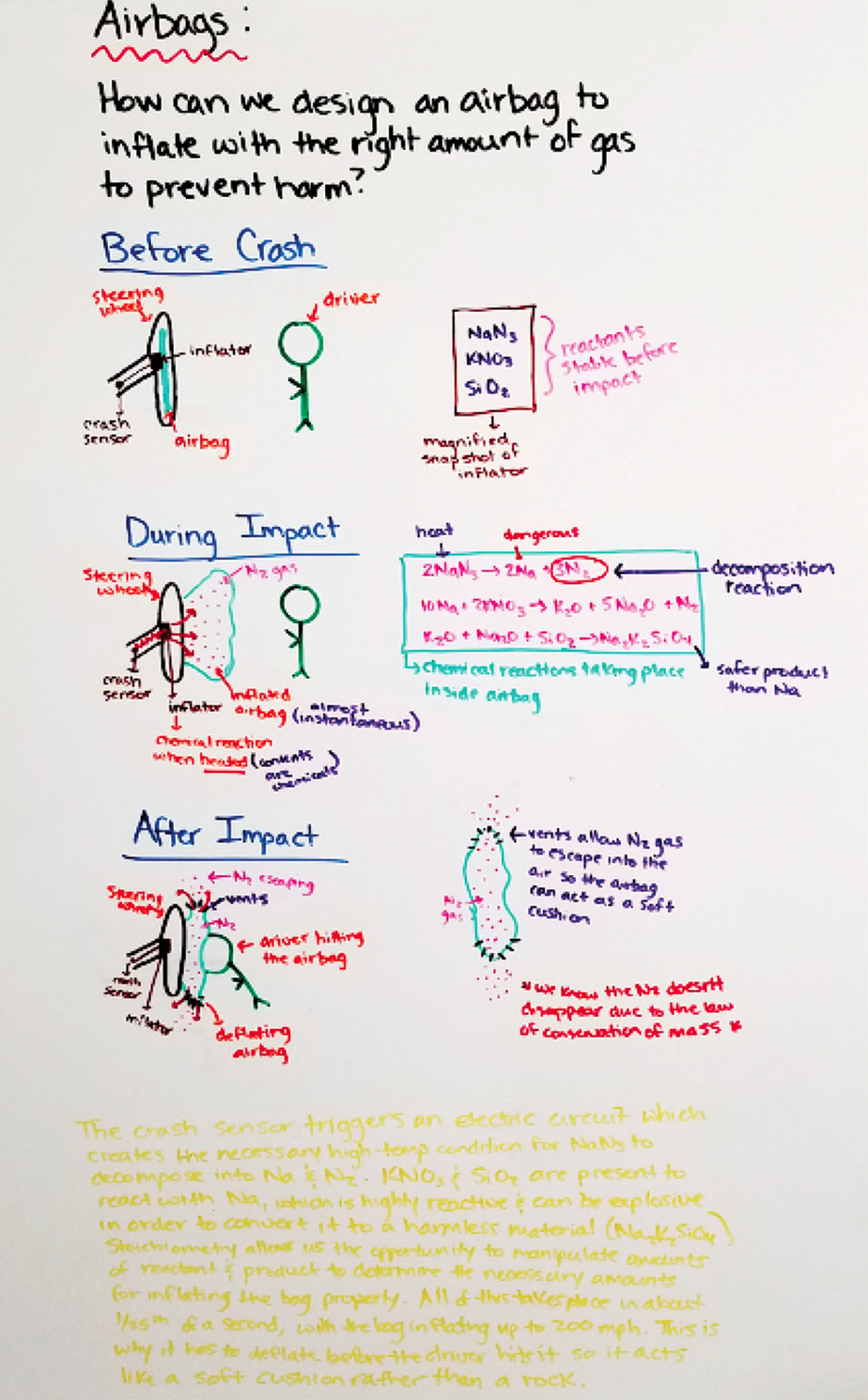
Example of final model.

Gotta Have Checklist for final models.
Students receive a grading rubric for their final model and evidence-based explanation at the beginning of the unit (see Online Resources, Table 5), which they use as a guide for constructing, revising, and finalizing their models throughout the unit. The end goal is to provide an explanation that reveals the story of what happened, the important science ideas necessary to explain mechanistically what happened, and evidence of how each aspect of the explanation is known.
Engineering design
Over the course of two days (two 50-minute class periods), we introduce students to the engineering design process, with the desired outcome of students designing a model airbag system in a prototype car that protects a raw egg in a simulated crash test. Although airbags are known to inflate from nitrogen gas produced by the explosive decomposition of sodium azide, we opted to represent this real-world reaction with safer, more accessible chemicals, sodium bicarbonate (baking soda) and acetic acid (distilled white vinegar). This task aligns with the characteristics of engineering proposed by Whitworth and Wheeler (2017); students are designing a solution to a problem, working under constraints, and are not provided with step-by-step instructions.
Before students officially begin the design process, we provide them with materials and design requirements (see Online Resources, Appendix A). During the planning stage, students brainstorm possible designs and methods for testing those designs in order to construct an airbag that inflates with the right amount of carbon dioxide to prevent harm to their vehicle occupant (raw egg dummy). We encourage students to use previous notes and assignments, public records, and knowledge gained throughout the previous unit to help approach the design task.
During the planning stage, students researched the safety hazards associated with the required chemicals, and recognized the potential threats of eye and skin irritation, as well as respiratory tract irritation should the baking soda or distilled white vinegar be mishandled or spilled. To address these safety concerns, students wear safety goggles and aprons throughout the design and testing process.
Before students begin constructing the model, make sure groups have a plan for collecting data and documenting evidence throughout the testing process. Additionally, students should be encouraged to use stoichiometric calculations as justification for their design choices. The teacher should give final approval to students’ designs before they advance to the construction and testing of their prototypes. At this point, the teacher should be checking for safety issues and confirming that the design meets the initial requirements, though it does not mean the design needs to be finalized at this stage.
Students are required to design, construct, and test at least two prototypes, but are given enough materials to construct four prototypes should they need more revisions. Once students complete the design challenge, they are evaluated based on their design, data collection, redesign, and justification for the prototype selected as the final submission (see Online Resources, Appendix A). Groups also develop evidence-based explanations to practice scientific writing and to prepare students for constructing their individual evidence-based explanations in their final unit plan models. After completing the engineering design task, students then complete a group and individual self-assessment (see Online Resources, Table 6).
Conclusion
MBI serves as a helpful instructional model developed by combining ideas of scientific and engineering practices to aid students in formulating questions and procedures, carrying out experiments, and communicating conclusions supported by evidence. The real-world context that relates to the anchoring phenomena used within MBI units provides a unique opportunity to provide students with an authentic learning experience in which they engage in engineering design to solve a real-world problem. ■
Online Resources
Table 1: https://bit.ly/3ovxRQO
Table 2: https://bit.ly/2Yuxtaq
Table 4: https://bit.ly/2KZKIgc
Table 5: https://bit.ly/3cocCha
Table 6: https://bit.ly/3iXDUfN
Appendix A: https://bit.ly/3coci1W
Ambitious Science Teaching: https://ambitiousscienceteaching.org/
MBI: https://sites.google.com/view/modelbasedinquiry/home?authuser=0
Airbags: Toyota Crash Tests (2:12) https://youtu.be/Bw0Ps8-KDlQ
How Do Airbags Work? (3:33) https://youtu.be/Y2sjYOGSV7E
How an Airbag Works: Takata Recall Explained (3:51) https://youtu.be/I_hkGN8TiJY
Carly A. Rock (carock@go.olemiss.edu) is a graduate student in chemistry and secondary education at The University of Mississippi in Oxford, MS. Brooke A. Whitworth (bwhitwo@clemson.edu) is an Associate Professor of Science Education at Clemson University in Clemson, SC.
Chemistry Engineering Inquiry NGSS Phenomena Physical Science Safety Science and Engineering Practices Three-Dimensional Learning High School



