feature
Science and Cooking
A fun approach to learning during teaching
The Science Teacher—September/October 2021 (Volume 89, Issue 1)
By Virginie Monnier and Magali Phaner-Goutorbe

Among the new educational tools and new ways of teaching/learning of the last 20 years, educational approaches based on constructivism are of great interest because they offer the possibility for students to build scientific concepts from experimental observations. The efficacy of these methods strongly depends on the choice of the initial topic provided by the teacher; it should, as much as possible, engage everyone, and deal with real people and real objects (Hendry 1996). Cooking is truly a universal topic—it can be considered a true multidisciplinary science as it incorporates chemistry; biology (Provost 2016); and to a lesser extent, physics. Therefore, it offers the possibility to perform many experiments and to evaluate them with all five senses, which is perfect for educational purposes, and in particular for children.
We introduce a fun approach, mixing science and cooking, that involves several kinds of students and teachers: engineering students (ages 20–22), cooking students (ages 18–25), secondary school students (ages 14–15), and their respective teachers. We have experimented over the last few years with several working themes, such as eggs, sugar, and chocolate. Here we report on using chocolate as the theme. After a presentation of the project and the lesson, the benefits and difficulties of this approach for the different participants will be discussed. A specific focus will be given to the engineering students, who were simultaneously teachers and learners.
Project objectives and organization
This project originates from teachers of an engineering school in which, after a three-year curriculum, students receive a general engineering degree. At the beginning of their first year, they are asked to select a project topic with a group of five or six students, to be worked on for nine months.
The title of the project presented and discussed in this article is: “Conception of scientific interactive experience on science and cooking for secondary school pupils.” The context of this project was to create a library of scientific experiments as educational supports. The main task was to present science in a more playful way, closer to real life than how it is traditionally presented at school. There were also multiple objectives: inform students about engineering and scientific jobs, encourage girls to choose engineering studies, and motivate underprivileged students to apply to universities or engineering schools. For this last objective, we established a partnership with a secondary school located in a “priority education area,” which means that students of this school face more social and academic difficulties than those from other schools.
As shown in Figure 1, many different students and teachers were involved in the project. The topic Science and Cooking was selected because cooking can be considered a real scientific field, and also because it is a universal and popular theme (note the explosion of television shows and movies concerning food and cooking in the past few years). The geographical proximity between the engineering school and a famous cooking school was also a real asset to start this project.
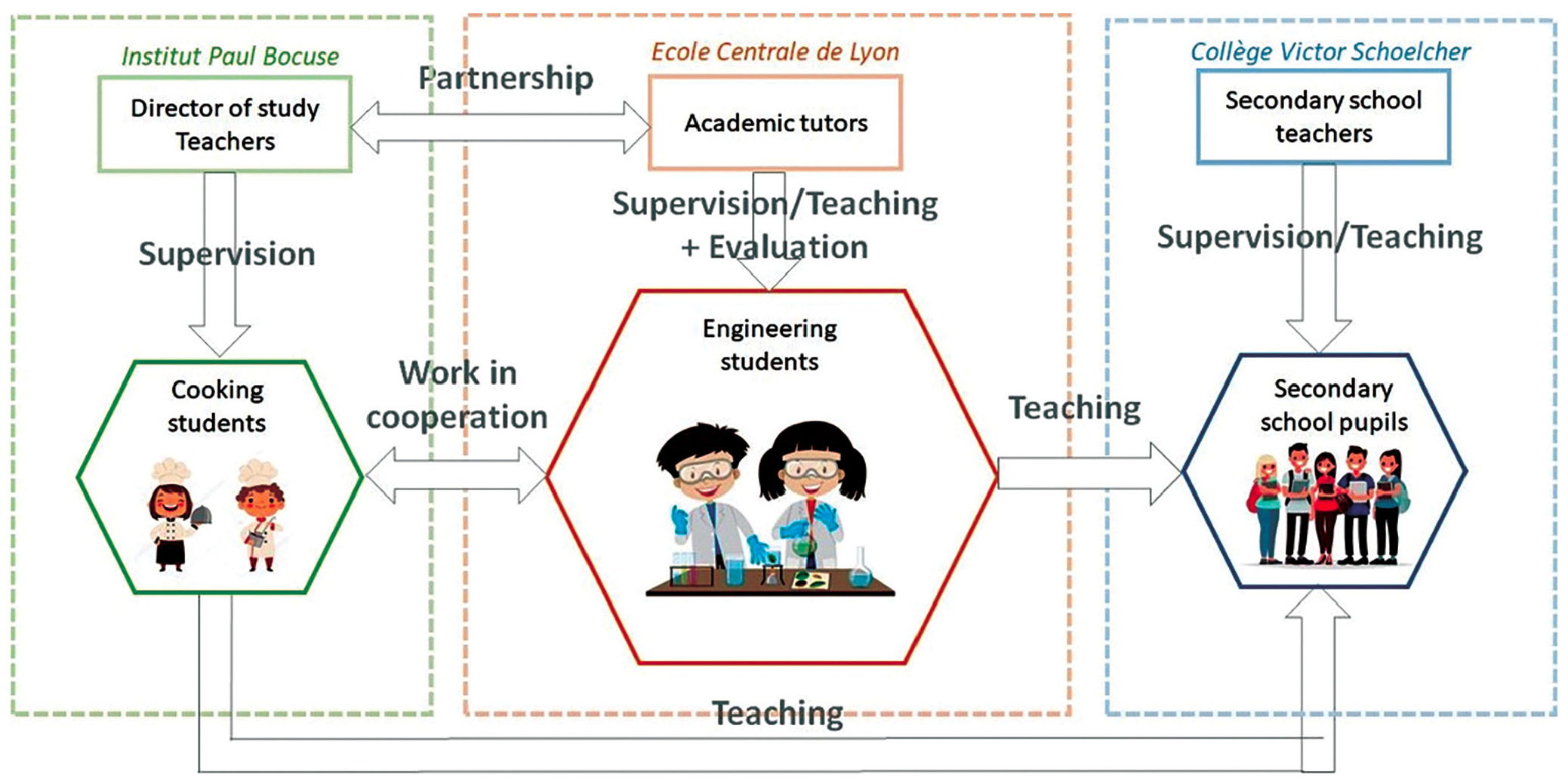
Relationships between the different actors involved in the project. As shown in this scheme, the analysis is focused on engineering students who are at the core of the project.
The project requires a lot of cooperation between the different groups involved. We supervised and evaluated the engineering students. To conceive and perform experiments, the engineering students worked with cooking students, who were supervised by the dean of education and chefs from the cooking school. Their common objective was to present experiments to secondary school pupils, who were supervised by three of their teachers (teachers in physics, chemistry, and mathematics).
Activity setup
Experiments were selected in alignment with the secondary school curriculum: Cooking was a pretext for engineering students to demonstrate complex scientific concepts to secondary school students. After discussion between engineering students, cooking students, and their academic advisors, chocolate was selected as a study topic. Why chocolate? It is a special treat, it is eaten all around the world, and almost everyone likes it. Therefore, it represents an attractive front door to introduce science.
At the scientific level, chocolate can be considered a complex food product, with many different components: cocoa, cocoa butter, sugar, lecithin, milk, etc. It also has specific mechanical properties (Diak 2006) and can be studied in materials science as an amorphous/crystalline compound (Afoakwa 2016). It can be used in a large range of experiments, and as a consequence many scientific concepts can be discussed (Beckett 2008). These scientific concepts were found to be appropriate to the academic level of the secondary school students and their physics and chemistry curriculum, the main objective of which is to understand the organization and transformation of matter.
Cooking recipes and scientific experiments were selected and designed following discussions between engineering and cooking students. The process required several steps:
- research to evaluate chocolate’s physical, chemical, and mechanical properties;
- selection of relevant experiments with the following criteria: fun, visual, scientific significance, and reliability;
- practical setup of experiments;
- testing compatibility with recipes that can be prepared by cooking students in class;
- in-depth learning of scientific concepts linked to the experiments;
- equation-free presentation for the secondary school audience;
- educational supports and event site preparation;
- rehearsal of the recipe/experiment in front of academic advisors;
- presentation of the final event; and
- assessment of students’ understanding.
Concerning step 2, numerous possible experiments were rejected. Although some experiments were visually impressive, the engineering students were not able to find any reliable scientific resource to explain them. In particular, this was the case for an experiment concerning “plastic chocolate” (cocoa butter than can be transformed to reach the same properties as modelling clay).
Some experiments were extremely interesting from a theoretical point of view, but impossible to demonstrate in class. Engineering students found recent scientific papers and patents about chocolate film mimicking plastic films using electrohydrodynamic spraying (Gorty and Barringer 2011), which might revolutionize the candy industry, or about a pulsed-laser technique to create drawings on chocolate (Kuwabara and Kuwabara 2007). However, their recent discovery and complex experimental setup made these experiments difficult to perform in front of secondary school students.
Safety was also considered; experiments were rejected due to the use of harmful chemicals that were incompatible with their use in the kitchen laboratories of the cooking school. The final criteria was that the experiments needed to be easily reproduced both in the classroom by teachers and at home by students.
The link between cooking recipes, scientific experiments, and scientific concepts
Table 1 summarizes the following cooking recipes and corresponding scientific experiments. Recipe/Experiment 1 deals with tempering and its link to the structural change of matter related to temperature. This experiment uses precise heating and cooling cycles in order to obtain chocolate with a brilliant finish and to avoid bleaching. Chocolate changes from an amorphous phase to a crystalline phase, with several polymorphs (i.e. several possible crystalline structures). The precise control of temperature is the key to get the desired crystalline structure and consequently the expected optical properties.
Recipe/Experiment 2 is dedicated to pastry decorations (such as lamellas, twists, etc.) made from chocolate. This is directly linked to the mechanical and rheological properties of chocolate, introducing the concepts of materials deformation and fracture. The brittleness of chocolate during tempering is due to the creation of microscopic defects in the solid structure. The influence of temperature on brittleness is demonstrated through the behavior of a chewy caramel candy (initially having plastic properties) after putting it in the refrigerator. This is similar to the rapid cooling of steel during its production.
Recipe/Experiment 3 presents the physical and chemical properties of an emulsion with the preparation of a recipe of mousse made only from chocolate. An emulsion is a heterogeneous mixture of two non-miscible liquids, which is kept stable by the introduction of a tensioactive, or amphiphilic, molecule. Chocolate itself has the necessary components to produce a stable emulsion if water is added—cocoa butter as fat matter and lecithin as a tensioactive molecule.
Recipe/experiment 3 will be explained in detail in the next section. Additionally, we showed a presentation called “Chocolate story” about the history of chocolate and the preparation process from cocoa bean in a lecture hall using Prezi software. A chocolate tasting session followed the presentation to introduce the perception of flavors.
Timing, grouping, and learning supports
Each recipe/experiment is designed to last 20 minutes. This duration was determined by the total time of the event (two hours) and the number of secondary school pupils involved (28). Students were divided into four groups of seven that moved from one recipe/experiment to the next. These small groups (instead of whole-class teaching) were preferred to facilitate interaction during recipes/experiments.
Various learning supports (posters, animated Power Point presentations, diagrams drawn on poster board, Prezi software for interactive presentations) were prepared and presented to the secondary school students. We wanted a variety of media to help the rhythm of presentations, to make them less monotonous, and to better catch the attention of the secondary students.
Lesson sequence: Chocolate Chantilly
This recipe introduces the scientific concepts of tensioactive molecules, hydrophilicity (which has affinity for water) or hydrophobicity (which avoids water), and lipophilicity (which has affinity for grease) or lipophobicity (which avoids grease). The concept of micelles as ultrafine droplets of fat in water is also presented to explain why the emulsion is stable and how it results in the mixing of two initially nonmiscible liquids.
Recipe/experiment 3 demonstrates the preparation of a whipped mousse of chocolate based on emulsion. This recipe, also called Chocolate Chantilly was first proposed by Hervé. This (This 1997), and consists of replacing eggs (from the traditional chocolate mousse recipe) with chocolate components themselves. Traditional chocolate mousse acquires air in its structure due to the presence of egg white. Bubbles are created during whipping (Figures 2a and 2b). They are stabilized by the formation of an air/water interface created by the proteins of egg white (This 2008): hydrophobic parts of proteins are preferentially exposed to air while hydrophilic parts stay in solution, which reduces the surface tension. Then the fat matter of chocolate (cocoa butter) is incorporated and stabilizes the preparation when it crystallizes inside the bubbles (Figure 2c).
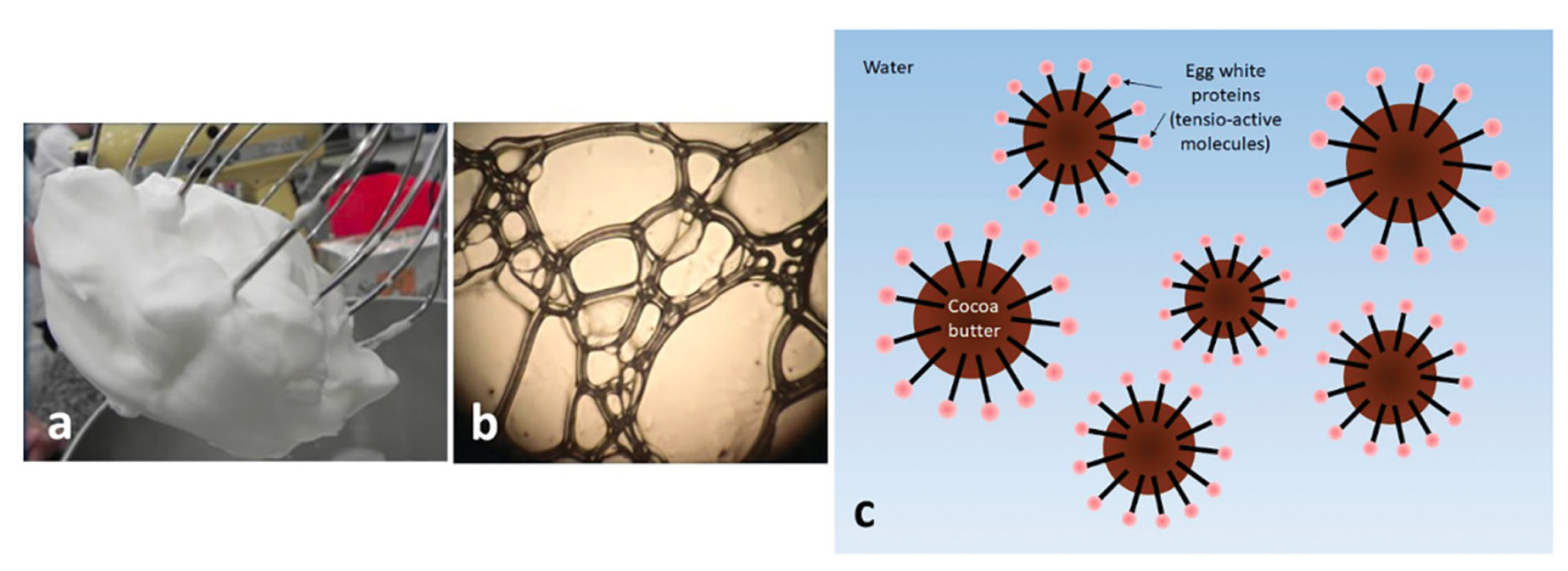
Photograph of beaten egg white with the naked eye (a) and under an optical microscope (b). Scheme of the resulting emulsion when cocoa butter crystallizes inside the air bubbles (c).
| Table 1. Link between recipes/experiments and scientific concepts. Educative supports used during their presentations. | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The objective of the experiment is to show that any kind of tensioactive molecule can replace the proteins of the egg white. In particular, lecithin (found in chocolate) can play this role. The new recipe consists of mixing water and chocolate to make an emulsion (Figure 3a) then whipping this emulsion on an ice bath to produce the mousse (Figure 3b). Lecithin stabilizes the emulsion, which is then whipped to incorporate air bubbles. During this step, the cooling from the ice bath crystallizes the cocoa butter, which stabilizes the air bubbles, just as in traditional chocolate mousse. The difficulty for the engineering students is to be able to present these concepts, which they already understand mathematically, to an audience that does not have this depth of knowledge. The secondary students’ experience and knowledge does not include equations to calculate the pressure inside an air bubble (Figure 4).

Photograph of the emulsion made with chocolate and water (a), Chocolate Chantilly obtained after whipping in an ice bath (b).
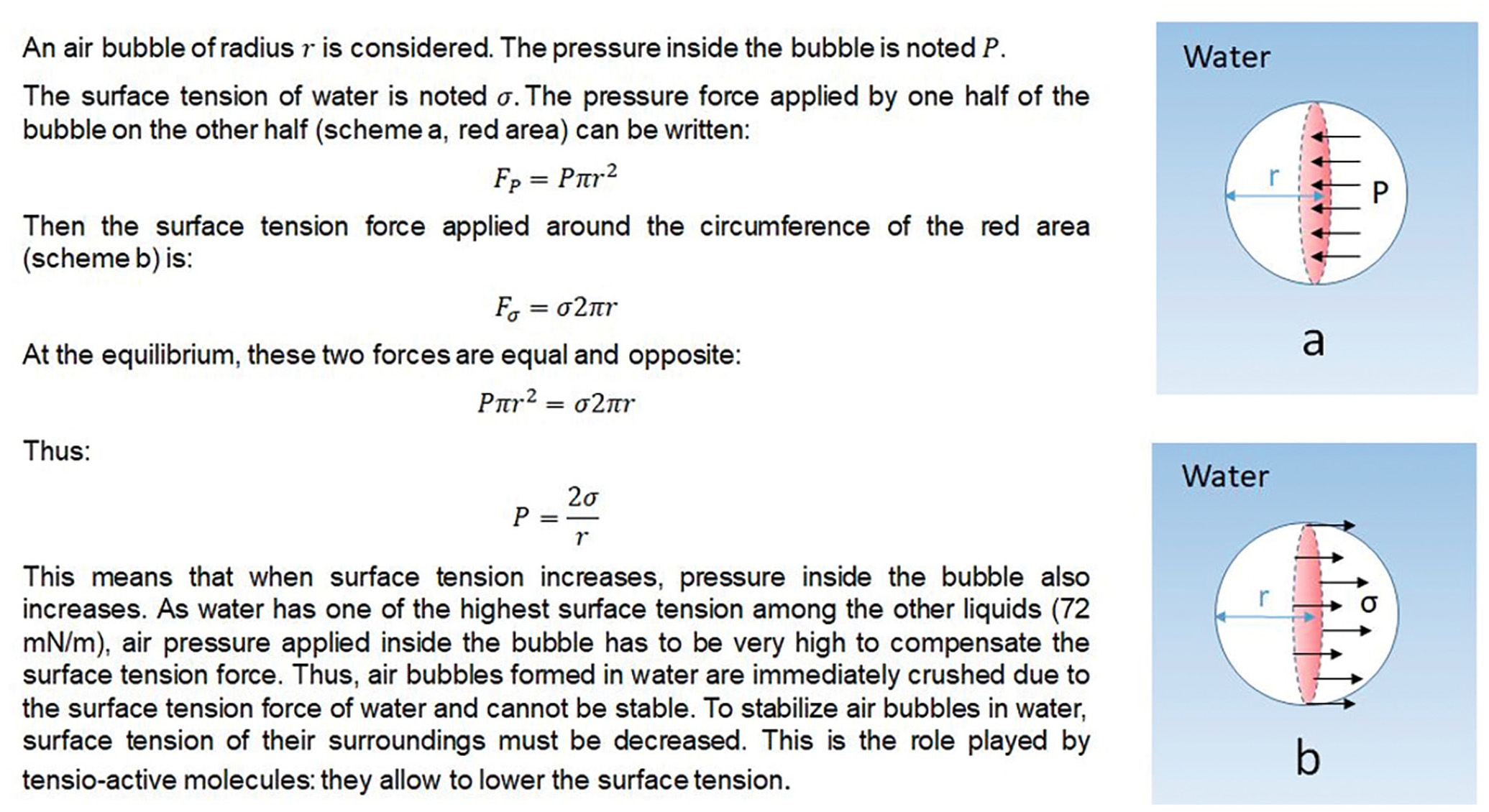
Scientific explanation and model taken for the study of pressure inside an air bubble of radius r in water: the pressure force (a) and the surface tension force (b) applied by one half of the bubble on the other half.
The engineering students first showed explanatory slides to the secondary school students, while cooking students made the Chocolate Chantilly, invited the students to mix water and oil, and asked them to try to whip the mixture into an emulsion (Figure 5a). Obviously, this did not work (Figure 5b), and the engineering students then asked questions about emulsion stability (Why can we not mix these two liquids? Why do the oil droplets immediately disappear?)

Photograph of a secondary school student trying to form an emulsion with only oil and water (a) and corresponding Power Point slide (b). Slides were translated from French to English.
Using the same model as this first experiment, the cooking students prepared traditional chocolate mousse (using egg whites). In parallel, the secondary students performed a negative control experiment, trying to form a mousse by whipping only water. This moment was chosen to introduce the concepts of surface tension (Figure 6a), hydrophilicity (lipophobicity), and hydrophobicity (lipophilicity), as shown in Figure 6b.
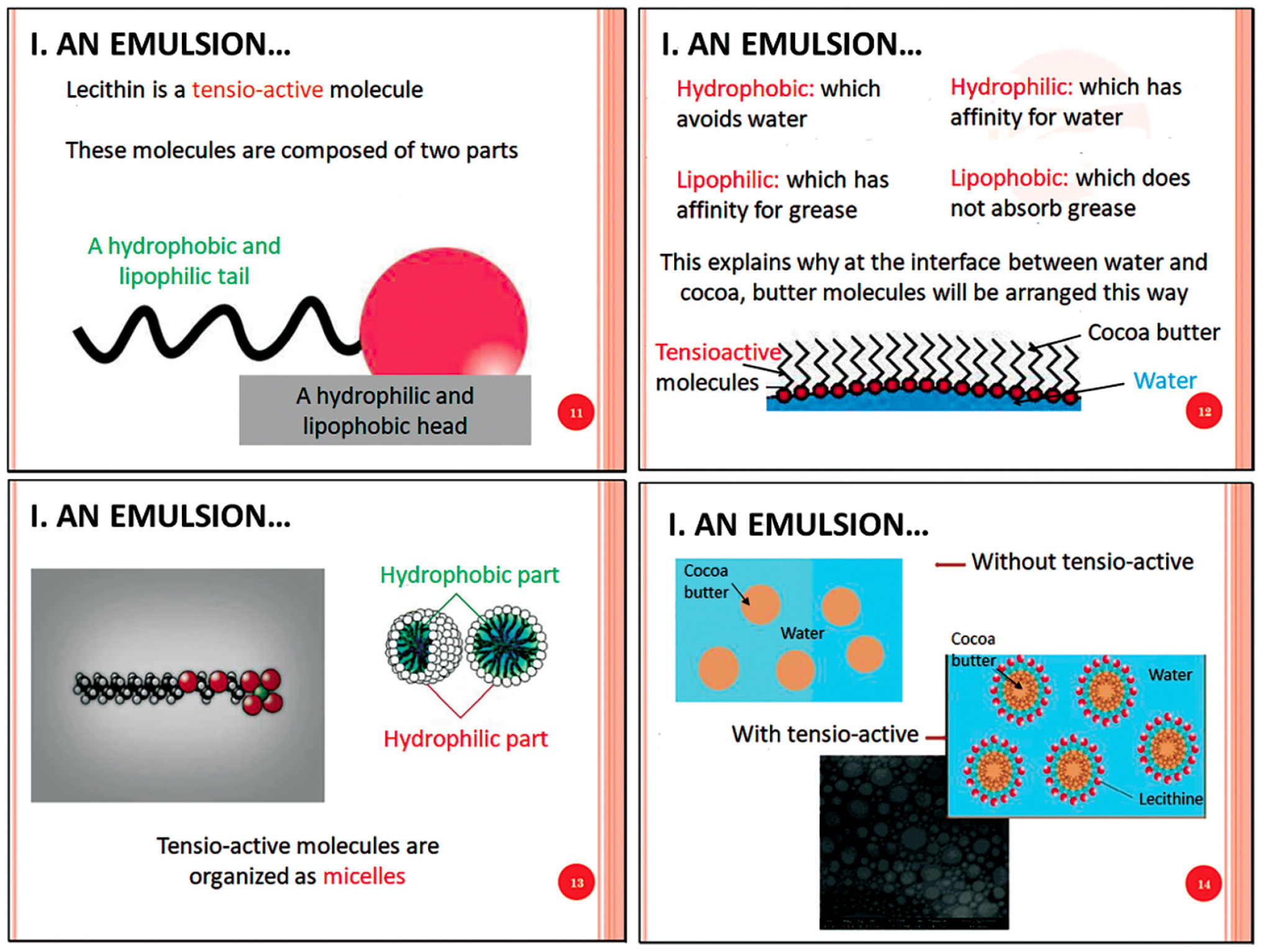
Slides corresponding to the tensio-active molecule concept presentation. Slides were translated from French to English.
We explained the concepts to secondary students using an analogy: Surface tension in mousse plays the same role as the surface pressure applied on the outside of a balloon (to balance the air pressure applied inside the balloon), as shown in Figures 7a and 7b. The surface tension of water is so high that it crushes the bubble right after its formation. The role of tensioactive molecules is to lower the surface tension, which can be achieved by adding some powder of soy lecithin to water. Once added, it was possible to produce a “water mousse,” as shown in Figure 8.
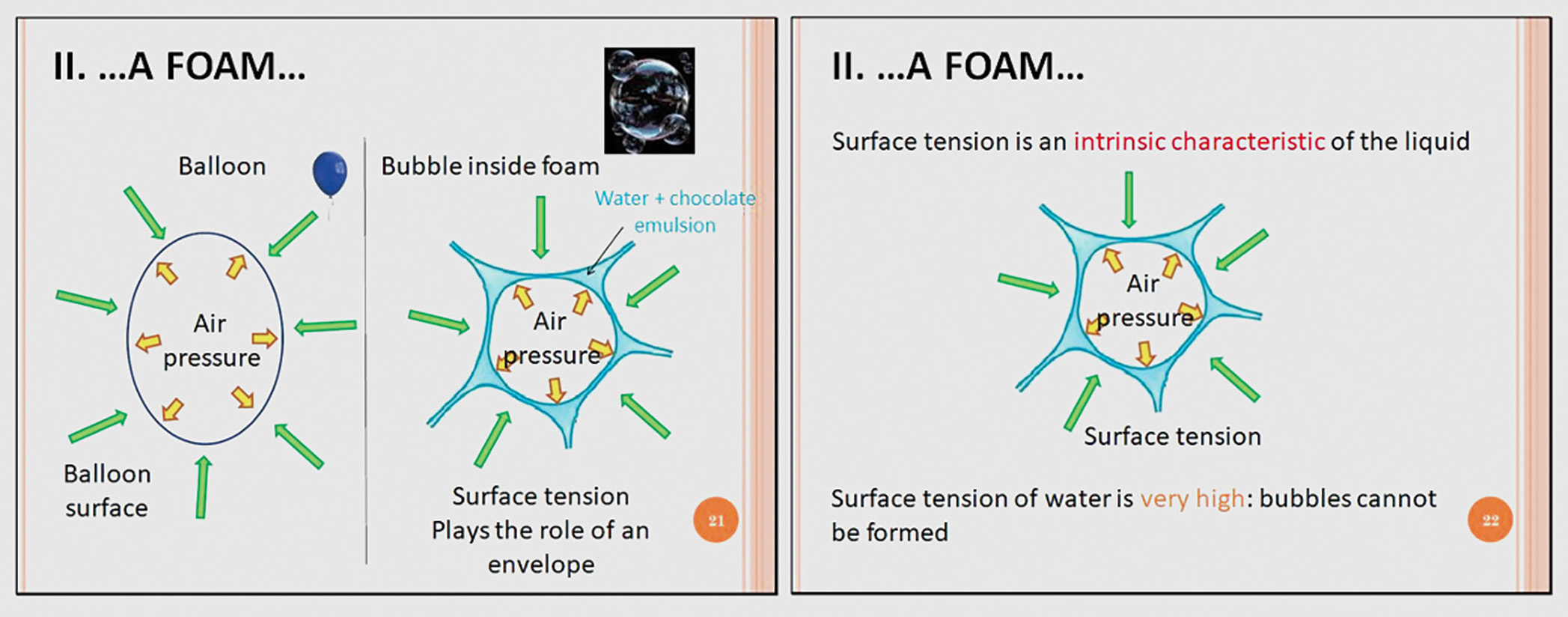
Slides explaining the surface tension concept. Slides were translated from French to English.

Photograph of the preparation of ‘water foam’ after soy lecithin addition.
At the end of the event, the secondary students completed a multiple-choice test containing three to four questions for each recipe/experiment (Figure 9). The main objective of this test was to reinforce the scientific vocabulary linked to this experiment. It also served as a way for engineering students to evaluate the secondary students’ understanding. These questions were included in a tear-off page of a booklet, summarizing the experiments, their main scientific concepts, and the corresponding recipes. The role of this booklet was not only to keep a record of the event, but also to be able to reproduce experiments and recipes at home or at school.

Multiple-choice test given to secondary school students at the end of the event.
Benefits of the project and relationship between engineering students and secondary students
The first goal of the project was to build interest in science among secondary school students ages 14–15 (last year of secondary school). This age group is critical; in France, after their final year of secondary school, students decide if they will continue (or stop) their academic studies. Secondary school is also a period during which most students show a decline in their attitude toward science, which is more pronounced for girls (Barmby 2008). In addition, the secondary school where we created the project is located in a less-affluent area of the city with lower-paying jobs, higher unemployment, and lower education; one of the objectives of this project is to introduce these students to young, successful students from other areas and encourage them to go on to further education.
During the event, students were enthusiastic and participated eagerly. Their opinions were recorded in an anonymous questionnaire, summarized in Figure 10. In the comments, most students said that they really liked the event because they ‘had fun.’ Some of them would have preferred to participate more in the experiments, doing more of the hands-on work themselves. This was difficult to achieve, however, due to safety rules and the number of students.
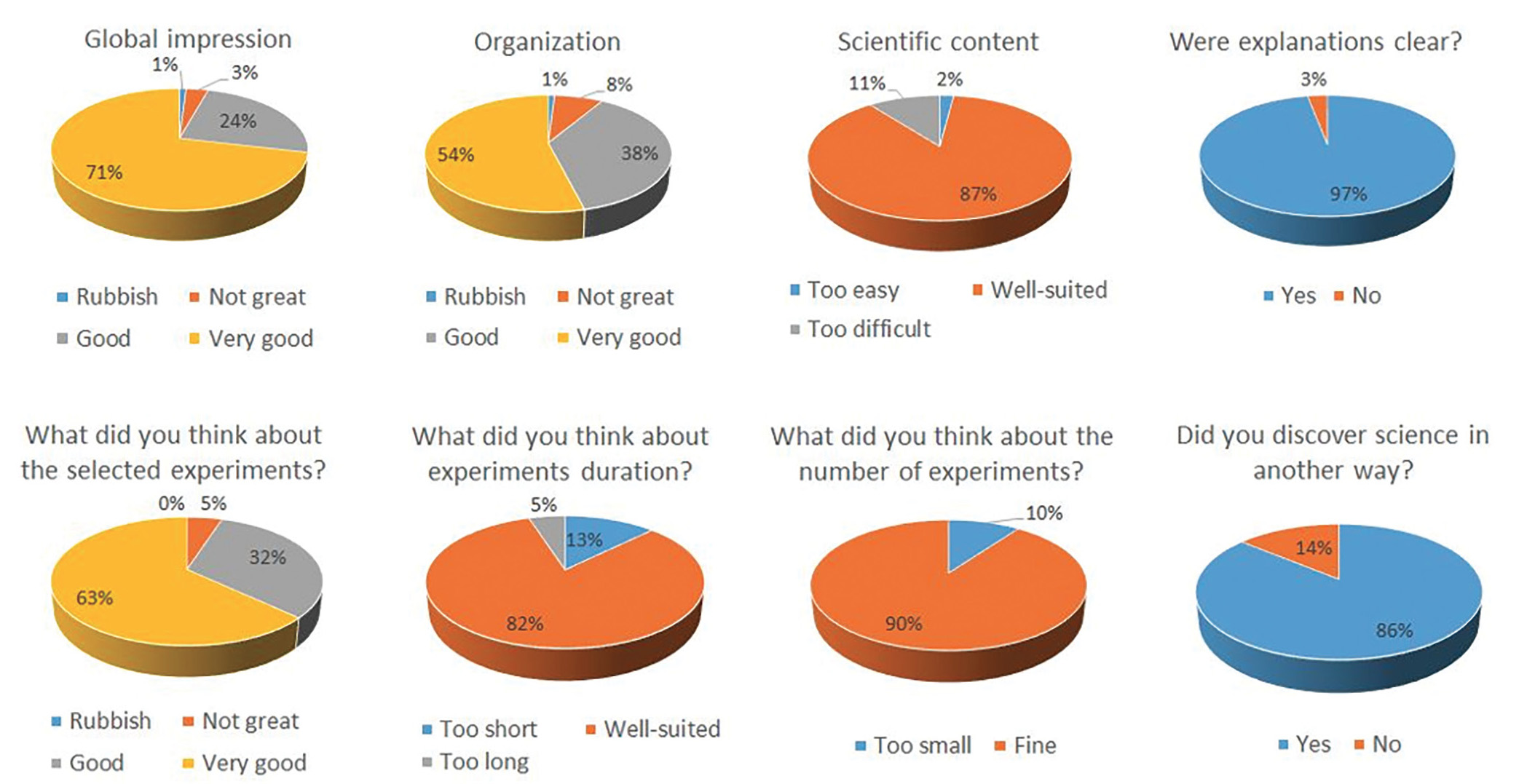
Results of the anonymous satisfaction questionnaire recording the opinion of secondary school students.
The engineering students were really satisfied with the behavior of secondary school students during the event. They noticed their attentiveness, curiosity, and relevant comments, which facilitated the scientific presentation.
After the event, a lunch was organized between all the participants, and we encouraged informal discussions between the secondary students and engineering students. The small age difference between secondary and engineering students (5–7 years) enabled discussion more easily; students asked many questions related to the engineering students’ studies and their future careers. This was also one of the objectives of our project, to present the engineering students as role models for the secondary students.
The project also helped the engineering students learn to work in project mode; introducing skills such as organization, team and project management, and teaching skills. As a group, they learned to manage tasks involving different groups and to manage a project during a long period (nine months) with a small budget (300 €). They also had to improve their communication skills between group members, supervising teachers, and partners.
One of the difficulties that engineering students encountered was supervision of the project, as evidenced by this student:
Even if this situation might not reappear in their future professional life, this is a really formative and useful experience, as a project manager should ideally know the work achieved by the team members in detail.
Another benefit to the engineering students was learning how to teach what they had learned about complex scientific concepts related to recipes (crystallization, surface tension) to secondary students. The engineering students had to modify their normal roles to play the part of teachers and to make difficult material understandable. One of the engineering students admitted:
Another student commented on the fact that learning how to explain and teach science will be a strong asset for his future job as an engineer:
The scientific supervisors organized several rehearsal sessions so that the engineering students could be ready for the event, which fundamentally improved the quality of presentations and enabled the engineering students to deliver the scientific message efficiently. Practicing also made the engineering students more confident for the event day. The importance of practice has also been shown to be effective in school-based teacher education (Buitink 2009).
Working closely with cooking students was crucial to the success of the project. According to the engineering students, working with cooking students was a privilege because they were cooking professionals, but it was also the cause of some difficulties. This project was an integral part of the engineering students’ curriculum, sessions were dedicated to the completion of the project on schedule, and grades would be assigned. For the cooking students, however, involvement in the project was totally voluntary. They had to find time to work on it, and to meet with the engineering students, which was mostly in the evenings, and was not always possible because they had to work in the school restaurant. Consequently, they were less involved than engineering students in the planning of recipes/experiments.
However, these organizational issues did not hinder the progress of the project too much. The main difficulty came from learning to communicate with people who approached the same subject from different perspectives. An example of this happened during one of the first meetings: cooking students were discussing flavors and visuals, while engineering students were speaking about physical and chemical properties of ingredients. Even if this way of working was first surprising, it became a great source of new ideas and knowledge.
Conclusion
Scientific experiments on cooking were performed thanks to the cooperation of engineering and cooking students. This project was achieved by student teams, mixing people with various sensitivities, knowledge, and skills. The main objective was to teach science in a fun way to secondary school students, many of whom dislike science and find it complicated due to mathematical equations. Cooking was selected as a universal theme, and the recipes/experiments are easy to reproduce without any science laboratory environment. Most of the secondary school students involved in this project really enjoyed it and found satisfaction in the fact that they were able to understand a concept, which seemed complicated at the beginning.
The secondary students were not the only learners in these experiments; the engineering students also acquired new skills in setting up experiments, preparing educational supports, and working with other professionals (the cooking students). This project also develops engineering students’ communication skills; in their careers they will have to be able to explain scientific concepts to non-experts such as bankers or decision makers. For all involved, this proved to be a successful and memorable event. ■
Virginie Monnier (virginie.monnier@ec-lyon.fr) is an associate professor of chemistry and Magali Phaner-Goutorbe (magali.phaner@ec-lyon.fr) is a professor of physics at École Centrale de Lyon, Ecully, France.
Careers Chemistry Curriculum Engineering General Science Interdisciplinary Labs Multicultural New Science Teachers Pedagogy Physical Science Physics Safety Science and Engineering Practices Teacher Preparation Teaching Strategies High School


